The Salmonellae constitute the most taxonomically complex group of bacteria among Enterobacteriaceae. Human Salmonella infections are most commonly caused by ingestion of food, water, or milk contaminated by human or animal excreta. Humans are the only reservoirs of S. Typhi. Four clinical types of Salmonella infections may be distinguished namely gastroenteritis, bacteremia or septicemia, enteric fever, and a carrier state. Of the various media employed for the isolation and preliminary identification of Salmonellae, particularly Salmonella Typhi; Bismuth Sulphite Agar (BSA) is the most productive. It is a modification of the original Wilson and Blair Medium for the isolation of Salmonella Typhi and other salmonellae and is particularly useful for the isolation of lactose-fermenting salmonellae.
Interesting Science Videos
Composition of Bismuth Sulphite Agar (BSA)
| Ingredients | Gms/liter |
| Peptone | 10.000 |
| HM Peptone B # | 5.000 |
| Dextrose (Glucose) | 5.000 |
| Disodium phosphate | 4.000 |
| Ferrous sulfate | 0.300 |
| Bismuth sulfite indicator | 8.000 |
| Brilliant green | 0.025 |
| Agar | 20.000 |
Final pH: 7.7±0.2
Principle of Bismuth Sulphite Agar (BSA)
Peptone and HM Peptone B serve as sources of carbon, nitrogen, long-chain amino acids, vitamins, and essential growth factors. Dextrose is the carbon source. Disodium phosphate maintains the osmotic equilibrium. Bismuth sulfite indicator along with brilliant green inhibits the intestinal gram-positive and gram-negative bacteria. Ferrous sulfate aids in the detection of hydrogen sulfide production. Clinical samples can be directly used to inoculate Bismuth Sulphite Agar. In the case of food samples, pre-enrichment of the sample is done prior to inoculation.
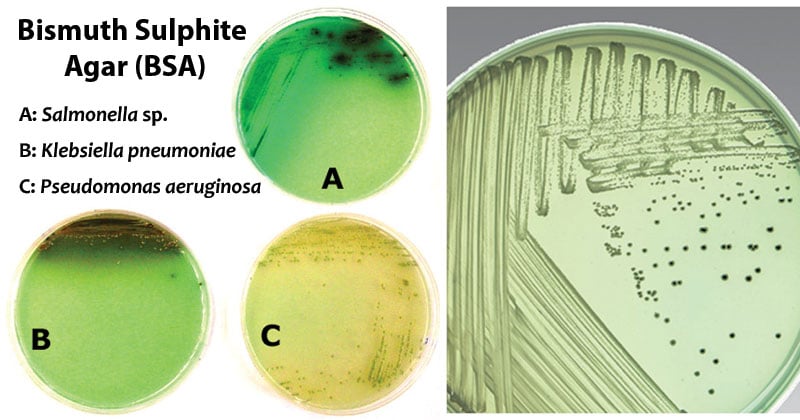
S. Typhi, S. Enteritidis, and S. Typhimurium typically grow as black colonies with a surrounding metallic sheen resulting from hydrogen sulfide production and reduction of sulfite to black ferric sulfide. Salmonella Paratyphi A grows as light green colonies.
Preparation and Method of Use of Bismuth Sulphite Agar (BSA)
- Suspend 52.33 grams in 1000 ml distilled water.
- Heat to boiling to dissolve the medium completely.
- DO NOT STERILIZE IN AUTOCLAVE or by fractional sterilization since overheating may destroy the selectivity of the medium.
- Mix well to disperse suspension and pour thick plates (25 ml medium per plate). Allow the medium to solidify with the dish uncovered.
- Dry the plates before use but take care to avoid over-drying.
- Correctly prepared plates should have a smooth, cream-like opacity with a pale straw color. There should be no sedimentation of the indicator.
- Inoculate the plates with the specimen.
- Incubate inoculated media for 48 hours at 35o Examine after 24 hours for typical colonies. If plates show little or no growth after 48 hours, incubate an additional 18-24 hours.
Note: The sensitivity of the medium depends largely upon uniform dispersion of precipitated bismuth sulfite in the final gel, which should be dispersed before pouring into sterile Petri plates.
Result Interpretation of Bismuth Sulphite Agar (BSA)
| Organisms | Growth |
| Enterobacter aerogenes | Poor growth; Brown-green (depends on the inoculum density) |
| Enterococcus faecalis | Inhibited |
| Escherichia coli | Poor growth; Brown-green (depends on the inoculum density) |
| Salmonella Enteritidis | Good-luxuriant growth; Black with a metallic sheen |
| Salmonella Typhi | Good-luxuriant growth; Black with a metallic sheen |
| Salmonella Typhimurium | Good-luxuriant growth; Black with a metallic sheen |
| Salmonella Abony | Good-luxuriant growth; Black with a metallic sheen |
Uses of Bismuth Sulphite Agar (BSA)
- Bismuth Sulphite Agar is recommended for the selective isolation and preliminary identification of Salmonella Typhi and other Salmonellae from pathological materials, sewage, water supplies, food etc.
Limitations of Bismuth Sulphite Agar (BSA)
- Bismuth Sulphite Agar may be inhibitory to some strains of Salmonella species and therefore should not be used as the sole selective medium for these organisms.
- Also, this medium favors the use of larger inoculum as compared to other selective media, as it has unique inhibitory action towards gram-positive organisms and coliforms.
- With certain Salmonella species, typical black colonies with a metallic sheen are observed near heavy inoculation and isolated colonies may show green colonies.
- Shigella species are mostly inhibited on this medium; exceptions being S. flexneri and S. sonnei.
- Some Salmonella like S. Sendai, S. Berta, S. Gallinarum, S. Abortus-equi are also inhibited.
