Interesting Science Videos
Ninhydrin Test Definition
Ninhydrin test is a chemical test performed to detect the presence of ammonia, primary/secondary amines, or amino acids. This test involves the addition of ninhydrin reagent to the test sample that results in the formation of deep blue color, often termed as Ruhemann’s purple, in the presence of an amino group.
Objectives of Ninhydrin Test
- To detect the presence of amines and amino groups in the test solution.
- To quantify the amino acids present in the sample.
- To distinguish carbohydrates from amino acids.
Principle of Ninhydrin Test
- This assay is based on the fact that two molecules of ninhydrin (2, 2- dihydroxyindane-1, 3-dione) react with a free alpha-amino acid to produce a deep purple or blue color known as Ruhemann’s purple.
- In this reaction, ninhydrin acts as an oxidizing agent and causes the deamination and decarboxylation of the amino acids at an elevated temperature.
- This reaction is then followed by condensation between the reduced ninhydrin molecules, released ammonia, and the second molecule of ninhydrin.
- By the end of the reaction, a diketohydrin complex is formed which has a deep purple color.
- In amino acids like proline and hydroxyproline, this test yields an iminium salt, which is yellow-orange in color.
- Similarly, proteins with a free amind group like asparagine, react with the ninhydrin reagent to form a brown colored product.
- The intensity of the formed complex is proportional to the concentration of amino acids in the solution.
- The color intensity, in turn, depends on the type of amino acid present.
Reaction
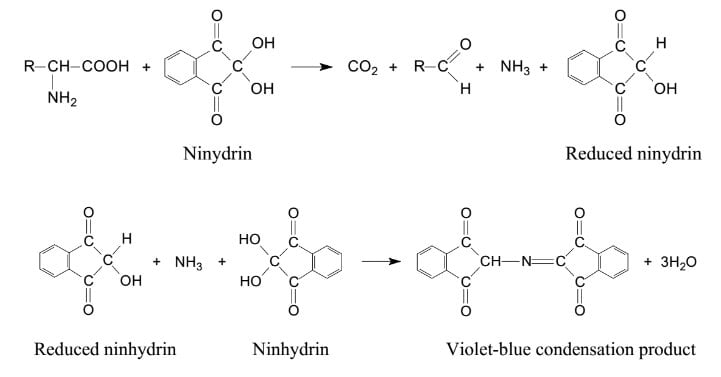
Image Source: Biocheminfo.
Requirements
Reagents
- Ninhydrin reagent: Dissolve 0.35g of ninhydrin in 100 ml ethanol (iso-propanol or 1:1 mixture of acetone/butanol may be used instead of ethanol).
- Diluent solvent (for the quantitative test): Mix equal volumes of water and n-propanol.
- Standard solution (1% protein solution)
- Sample solution
Materials required
- Test tubes
- Test tube stand
- Pipette
Equipment
- Water bath
- Spectrophotometer
Procedure of Ninhydrin Test
For qualitative analysis
- Take 1 ml of standard protein solution in one test tube and 1 ml of the test sample in another dry test tube.
- Add a few drops of ninhydrin reagent to both the test tubes.
- Place the test tubes in the water bath for 5 minutes and then allow cooling to room temperature.
- Observe the formation of color and note down the result.
For quantitative analysis
- Pipette out different volumes (10 µl, 20 µl, and so on) of the protein solution from the supplied stock solution into a series of test tubes and make up the volume to 1 mL with distilled water.
- Take a tube labeled as one as blank containing 1ml of just distilled water and the rest of the tubes labeled 2 to 9 for construction of a standard curve. Tubes 10-15 are for the unknown samples.
- Add 1 ml of the ninhydrin reagent and 5 ml of diluent solvent to each tube and mix well by vortexing.
- Cool the tubes.
- Cover the tubes with caps on top and incubate at 90°C for 17 minutes or boiling water bath for 20 minutes.
- Cool the tubes to room temperature and measure the optical density of the solutions at 570 nm (440 nm for proline and hydroxyproline) against a blank.
- Prepare a standard curve of absorbance against amino acid concentration.
- Determine the amount of amino acid in the unknown sample by plotting a standard curve of A570 on the Y-axis and concentration of amino acid on the X-axis.
Result and Interpretation of Ninhydrin Test
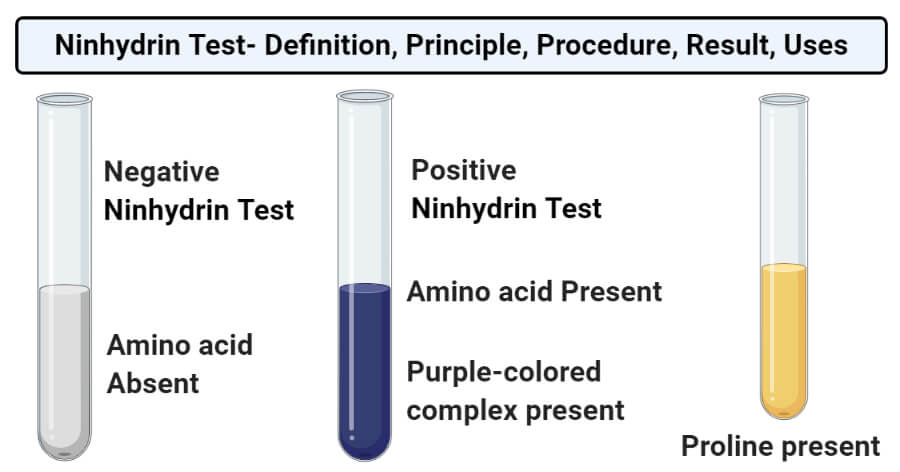
- The presence of a purple-colored complex in the tube represents a positive result and indicates the presence of amino acid in the sample.
- The absence of the complex in the tube represents a negative result and indicates the lack of amino acids in the sample.
- From the graph, we can determine the concentration of unknown samples.
Uses of Ninhydrin Test
- Ninhydrin test is used to detect the presence of amino acids in unknown samples.
- This test is also used in solid-phase peptide synthesis to monitor the protection for amino acid analysis of proteins.
- As the ninhydrin test is quite sensitive, it is commonly used to detect fingerprints. It is possible as the terminal amines of lysine residues in peptides and proteins shed off in fingerprints react with ninhydrin.
Limitations of Ninhydrin Test
- Ninhydrin reacts not only reacts with α-amino groups but also with nitrogen in ammonia and other free amines.
- The Ninhydrin test is not effective to detect high molecular weight proteins as the steric hindrance limits the ninhydrin from reaching the α-amino groups.
References and Sources
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- 4% – https://microbenotes.com/bials-test/
- 3% – https://en.wikipedia.org/wiki/Ninhydrin
- 2% – https://www.answers.com/Q/Account_for_the_formation_of_precipitate_in_the_test_for_purines.
- 1% – https://vlab.amrita.edu/?sub=3&brch=63&sim=1094&cnt=1
- 1% – https://en.wikipedia.org/wiki/Ninhydrin_assay
- 1% – https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch19/oxred_3.php
- 1% – https://breakingbiochem.wordpress.com/tag/tests-for-amino-acids/
- 1% – https://biochemden.com/anthrone-method-carbohydrate-determination/
- 1% – https://answers.yahoo.com/question/index?qid=20060922055141AADsDD1
- <1% – https://www.ukessays.com/essays/biology/quantitative-tests-for-amino-acids-and-proteins-biology-essay.php
- <1% – https://www.epa.gov/sites/production/files/2015-08/documents/method_365-3_1978.pdf
- <1% – http://www.jbc.org/content/200/2/803.full.pdf
- <1% – http://www.allometric.com/tom/courses/bil255/bil255goods/03_proteins.html
- <1% – http://resources.schoolscience.co.uk/Unilever/16-18/proteins/Protch2pg5.html
- <1% – http://nobel.scas.bcit.ca/courses/wpmu/chem2204/files/2011/01/Techniques_and_Practice_3.pdf

How is ninhydrin test applicable in food industry?
What are it’s application in food industry?
will the following give a positive test for nihydrin test
>GELATIN
>GLYCINE
>LEUCINE
>ALBUMIN
>CASSEINE HYDROLYSYLASE
I did a lab test on some of these.
Gelatin turned out yellow-ish which indicates a positive test for proline, but negative for amino acids.
Glycine colored purple which is positive for amino acids.
Albumin colored yellow and is positive for proline.
I did not test Casseine, but it contains proline amino acids so i would say it would color yellow.
Glycine
thanks for the literature that is very educative