Starch is the reserved food material of most plants and is one of the abundant carbohydrates in nature. Starch is a polysaccharide made of two glucose polymers –amylose and amylopectin. Being a large macromolecule, starch can’t be used in its native state by microorganisms; hence it must be broken down into glucose before metabolism. Bacterial extracellular amylase enzyme can hydrolyze starch into maltose and glucose. However, not all bacteria are capable of producing amylase enzymes.
The starch hydrolysis test, also known as the amylase test, is a biochemical test that is used to determine the ability of bacteria (microorganisms) to produce amylase and utilize starch as a carbon source. It is commonly used to differentiate species of Bacillus, Clostridium, Corynebacterium, Fusobacterium, Streptococcus, Enterococcus, and Pseudomonas genus.
Interesting Science Videos
Objectives of Starch Hydrolysis Test
- To determine the ability of bacteria to produce amylase enzyme
- To determine the ability of bacteria to hydrolyze starch
- To differentiate and identify bacteria based on their ability to hydrolyze starch
Principle of Starch Hydrolysis Test
- Bacteria capable of producing extracellular amylase enzymes can hydrolyze the starch and produce smaller water-soluble sugar molecules like glucose, maltose, and dextrose. If the bacteria are not able to secret the amylase enzyme, there won’t be hydrolysis of starch.
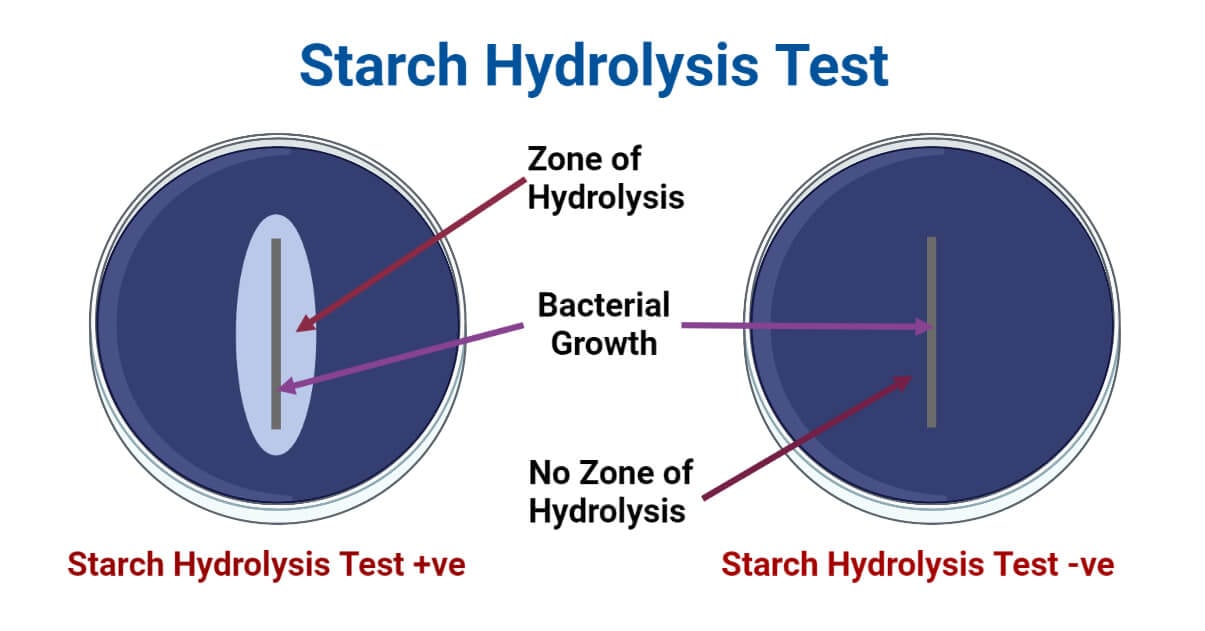
- If the inoculated bacteria produce amylase, the starch molecules around the colonies will be hydrolyzed and there won’t be amylose or starch molecules. Hence, upon the addition of iodine solution, there won’t be the formation of dark blue color around the colonies.
- If the inoculated bacteria produce amylase, starch molecules around the bacterial colony will be hydrolyzed; so there won’t be color development in the medium surrounding the colony upon the addition of iodine solution.
- Thus, hydrolysis of starch can be identified by the formation of a clear zone of halo around the bacterial growth after the addition of iodine solution in the inoculated and incubated starch medium.
Requirement for Starch Hydrolysis Test
1. Culture Media
Mueller Hinton Agar (MHA), Starch Agar, and Heart Infusion Agar with 2% Starch are commonly used for the detection of starch hydrolysis.
Composition of Mueller Hinton Agar per 1000 mL
HM Infusion B form (Beef Infusion) 300.00 grams
Acicase (Casein Acid Hydrolysate) 17.500 grams
Starch 1.5000 grams
Agar 17.000 grams
Final pH 7.3 0.1 at 250C
Reference: SM173.pdf (himedialabs.com)
Preparation of MH Agar
- Measure the appropriate amount of MHA media powder (38.0 grams per 1000 mL) and dissolve it in the water of the required volume in a conical flask (or glass bottle).
- Stir well in a magnetic stirrer or manually and heat to boil to completely dissolve the media powder.
- Autoclave the medium at 121°C and 15 lbs. pressure for 15 minutes.
- When the medium is cooled to around 40 to 45°C, in a sterile petri dish (of 10 cm diameter) dispense about 20 to 25 mL of the medium and let it solidify.
Composition of Starch Agar per 1000 mL
Meat Extract- 3.00 grams
Peptic Digest of Animal Tissue- 5.00 grams
Starch- 2.00 grams
Agar- 15.0 grams
Final pH- 7.2 0.1 at 25°C
Reference: M107S.pdf (himedialabs.com)
Preparation of Starch Agar
- Measure the appropriate amount of Starch Agar media powder (25.0 grams per 1000 mL) and dissolve it in the water of the required volume in a conical flask (or glass bottle).
- Stir well in a magnetic stirrer or manually and heat to boil to completely dissolve the media powder.
- Autoclave the medium at 121°C and 15 lbs. pressure for 15 minutes.
- When the medium is cooled to around 40 to 45°C, in a sterile petri dish (of 10 cm diameter) dispense about 20 to 25 mL of the medium and let it solidify.
2. Reagents
Gram’s Iodine Solution is required for the detection of starch hydrolysis.
Preparation of Gram’s Iodine Solution:
- Dissolve 25 grams of Iodine Crystal and 50 grams of potassium iodide in 500 mL of distilled water to make Lugol’s iodine stock solution.
- Dissolve 5 grams of sodium bicarbonate (NaHCO3) in 100 mL of distilled water to make 5% sodium bicarbonate solution.
- Mix 60 mL of Lugol’s iodine solution and 60 mL of 5% sodium bicarbonate solution with 220 mL of distilled water to make Gram’s Iodine Solution.
(Reference: Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC: ASM Press 1752 N St., N.W., [2016] doi:10.1128/9781555818814.ch3.17.33. Appendix 3.2.1-1; Preparation of Gram Stain Reagents)
Alternatively:
- Dissolve 1.0 grams of iodine and 2.0 mL gram of potassium iodide in 300 mL of distilled water.
(Reference: S013.pdf (himedialabs.com))
3. Equipment
| Petri Plate Incubator | Dropper Autoclave | Bunsen burner Weighing Machine | Inoculating loop |
4. Test Organism (Sample Bacteria)
5. Control Organisms
Bacillus cereus ATCC – 10876
Escherichia coli ATCC – 25922
Streptococcus bovis ATCC – 33317
Staphylococcus aureus ATCC – 25923
Procedure of Starch Hydrolysis Test
- Using a sterile inoculating loop (or cotton swab) pick up the sample bacteria from several fresh colonies (of about 18 to 20 hours).
- Streak the bacteria in the form of short and thick straight lines over the surface of the bacteria. (Multiple bacteria can be tested in a single plate forming multiple lines.)
- Incubate at 35±2°C for at least 48 hours.
- Following incubation, add a few drops of iodine solution directly over the colonies and observe for the formation of a clear halo around the colonies.
Result and Interpretation of Starch Hydrolysis Test
- A positive result is indicated by the formation of a clear halo around the colonies and the development of dark blue to purple-blue color in the surrounding medium after the addition of Gram’s Iodine.
- A negative result is indicated by no clear halo around the colonies and the development of dark blue to purple-blue color in the surrounding medium after the addition of Gram’s Iodine.
Starch Hydrolysis Test Results of Some Common Bacterial Pathogens
- Starch hydrolyzing (amylase producing) bacteria: S. bovis, Bacillus subtilis, B. cereus, B. megaterium, Clostridium perfringens, etc.
- Starch non-hydrolyzing (amylase non-producing) bacteria: Corynebacterium diphtheria, Clostridium difficile, C. botulinum, bile-esculin positive viridans Streptococci except S. bovis, E. coli, S. aureus, Pseudomonas aeruginosa, P. putida, etc.
Quality Control
- Bacillus cereus ATCC – 10876 and Streptococcus bovis ATCC – 33317 gives positive result i.e. formation of a clear halo around their colonies is observed after the addition of the Gram’s iodine while the rest of the medium turns dark blue colored.
- E. coli ATCC – 25922 and Staphylococcus aureus ATCC – 25923 gives negative result i.e. don’t form a clear halo around their colonies but the surrounding medium turns dark blue after the addition of the Gram’s iodine solution.
Precautions
- Add Gram’s iodine only after 48 hours of incubation. Testing earlier may give a false negative result.
- While using multiple specimens in a single plate, make sure to streak colonies apart from each other making a gap of about 25 mm or more.
- Don’t use a medium containing a high amount of glucose because bacteria may use glucose instead of starch and give false negative results.
- Read the result quickly after the addition of iodine solution because the blue color may fade as time passes.
Applications of Starch Hydrolysis Test
- To differentiate species of Bacillus, Clostridium, Corynebacterium, Fusobacterium, Streptococcus, Enterococcus, and Pseudomonas genus.
- To separate S. bovis from other Bile-esculin positive viridans Streptococci.
- To separate Chryseobacetrium indologenes from Elizabethkinga meningoseptica; earlier positive while later negative.
Limitations of Starch Hydrolysis Test
- It is not a confirmatory test and hence requires other biochemical test results to completely identify isolated bacteria.
- It requires a longer time period (at least 48 hours).
- Once Gram’s iodine is added over the medium, bacteria can’t be used for further culture or other tests from the plate.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition) . Washington, DC : ASM Press 1752 N St., N.W., [2016] doi:10.1128/9781555818814.ch3.17.33
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.) P. 185 – 187. St. Louis, Missouri: Elsevier
- https://microbiologyinfo.com/starch-hydrolysis-test/
- https://www.uwyo.edu/molb2021/additional_info/summ_biochem/starch.html
- https://microbeonline.com/starch-hydrolysis-test/
- https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.17%3A_Starch_Hydrolysis
- https://microbiologynote.com/starch-hydrolysis-test/
- https://www.narajolerajcollege.ac.in/document/sub_page/20210610_233752.pdf
- https://asm.org/ASM/media/Protocol-Images/Starch-Agar-Protocol.pdf?ext=.pdf
- https://www.deshbandhucollege.ac.in/pdf/resources/1586748276_BT(H)-VI-IEM-Hydrolysis_of_starch_by_microorganisms.pdf
- https://www.sas.upenn.edu/LabManuals/biol275/Table_of_Contents_files/21-DiagnosticTests.pdf
- S013.pdf (himedialabs.com))
- M107S.pdf (himedialabs.com)
- SM173.pdf (himedialabs.com)

I was able to get all the information i need for my work but I didnt come across the apparatus and safety measures which was put in place for the lab work.