Interesting Science Videos
What is Staphylococcus saprophyticus?
Staphylococcus saprophyticus is a Gram-positive, coagulase-negative coccus that is an important part of the group of microorganisms causing urinary tract infections (UTIs), particularly in young sexually active females.
- Like all Staphylococci, S. saprophyticus is also clustering Gram-positive cocci, nonmotile, non-spore-forming, and facultatively anaerobic.
- S. saprophyticus is a part of the normal flora in humans that colonizes the areas like the perineum, rectum, urethra, cervix, and gastrointestinal tract.
- This bacterium is also a common gastrointestinal flora in pigs and cows and may be transferred to humans by eating these respective foods.
- Besides humans, it can also be isolated from other environmental sources like meat and dairy products.
- It is the second most common cause of UTIs in young and middle-aged women, next to Escherichia coli. Less commonly, it is also responsible for complications like acute pyelonephritis, urethritis, epididymitis, and prostatitis.
- Among staphylococci, S. saprophyticus is the only species that is typically uropathogenic which is due to its ability to adhere to uroepithelial cells and persistently grow in the urinary tract.
- S. saprophyticus is different from S. aureus in that it is coagulase-negative, meaning it lacks the enzyme coagulase. It can be differentiated from other coagulase-negative staphylococci by its resistance to Novobiocin.
- It was first isolated from humans by Shaw in 1951, and Torres Pereira first identified its connection with UTIs in 1962.
Classification of Staphylococcus saprophyticus
- Classification of different species of the genus Staphylococci is based on various factors ranging from morphology, chemical properties, amino acid sequences, biochemical characteristics, and nucleotide sequences.
- Staphylococcus spp. are primarily classified on the basis of DNA–DNA hybridization where members of the same species demonstrate relative DNA-binding values of generally 70 percent or greater.
- Staphylococcal subspecies, in turn, have been identified on the basis of phenotypic characters and DNA relatedness, and ribotyping.
There are two known subspecies of S. saprophyticus; S. s. saprophyticus and S. s. bovis which are differentiated based on their colony diameter and nitrate reduction activity.
| Domain: | Bacteria |
| Phylum: | Firmicutes |
| Class: | Bacilli |
| Order: | Bacillales |
| Family: | Staphylococcaceae |
| Genus: | Staphylococcus |
| Species: | S. saprophyticus |
| Subspecies: | S. saprophyticus subsp. saprophyticus |
| Subspecies: | S. saprophyticus subsp. bovis |
Habitat of Staphylococcus saprophyticus
- S. saprophyticus subsp. saprophyticus is present as the normal flora in most primates and smaller terrestrial animals in the Order Scandentia. Member of Rodentia might act as a temporary host.
- S. s. bovis, however, is found as the normal flora in domestic cattle like cows and pigs.
- S. s. saprophyticus is also present in humans as the normal flora of the perineum, rectum, urethra, cervix, and gastrointestinal tract.
- Besides being a part of the human microbiota, this species is widely distributed in the environments and has been isolated from cheese, meat, and raw milk. Also, reports of S. saprophyticus in the marine environment and food derived from fish have been made.
- Furthermore, S. saprophyticus has also been isolated from the marine environment, either in polluted or recreational waters.
- In humans, S. saprophyticus is sometimes found in the inguinal and perineal area that is characterized by high humidity, a rich supply of nutrients, near-body temperature, and a higher pH than is found on the general skin surface, factors that enhance its growth.
- The exact physiological role of the bacterium as the normal flora is not yet known; however, it is assumed that it may be involved in the lipid metabolism of the skin and that it may serve as a primary barrier against invading microbial pathogens.
Morphology of Staphylococcus saprophyticus
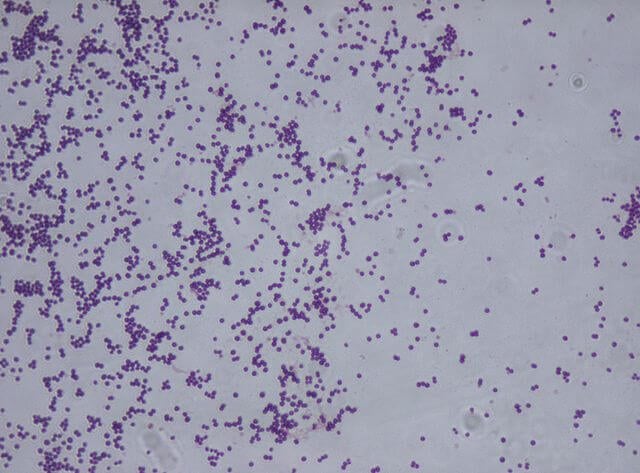
Figure: Gram Staining of Staphylococcus saprophyticus (Gram-positive). Image Source: Riraq25 (Wikipedia).
- Cells of S. saprophyticus are gram-positive cocci, nonmotile, non-spore-forming with an average diameter of 0.8 to 1.2 µm.
- These occur predominantly in pairs or as loose aggregates of single cells as a result of their division in irregular planes.
- The cell membrane is made up of the typical lipid-protein bilayer consisting of phospholipids and different proteins that together act as a selective barrier and fulfill functions like electron transport, active transport, septum formation, and segregation of DNA.
- The cell wall is made up of peptidoglycan and teichoic acid that function to maintain the spherical shape of the cell.
- Like other coagulase-negative Staphylococci, S. saprophyticus also has fewer cell wall adhesions and cell-wall associated proteins.
- S. saprophyticus have hemagglutinin mediated by its cell wall-anchored or associated surface proteins that help the organism anchor to the urogenital cells.
- It also has abundant transporter systems to adapt to ever-changing pH, osmolarity, and concentration of urea in human urine.
Cultural Characteristics of Staphylococcus saprophyticus
- Staphylococci produce different distinct colonies on a variety of commercial, selective, and nonselective agar media.
- The commonly used selective media include mannitol–salt agar, lipase–salt–mannitol agar, phenyl ethyl alcohol agar, Columbia colistin–nalidixic acid (CNA) agar, and Tryptic soy agar supplemented with egg yolk tellurite enrichment.
- These media inhibit the growth of gram-negative bacteria, but allow the growth of staphylococci and certain other gram-positive bacteria.
- The choice of media also depends on the source or sample of the infection. For food or other environmental specimens, Mannitol salt agar is used along with selective broth consisting of tryptone, nalidixic acid, and novobiocin.
- For human samples like urine, blood agar is used.
Figure: Staphylococcus saprophyticus on chromogenic agar (light pink) and blood agar (creamy bright). Image Source: Rich Davis.
1. Nutrient Agar (NA)
- Circular, cream-colored to white colonies of S. saprophyticus are observed on NA. The colonies are mostly 1mm in diameter with an entire margin.
- The colonies have raised elevation and a dense center with transparent borders.
- The anaerobic species of S. saprophyticus grow well under anaerobic conditions but grow very poorly under aerobic conditions.
2. Mannitol Agar
- Yellow-colored colonies indicating mannitol fermentation as the color of the media is converted from red to yellow.
- The colonies are 1-2 mm in diameter with an entire margin.
3. Blood agar (BA)
- Creamy Bright white non-hemolytic colonies, around 5-8 mm in diameter are seen on BA.
- Some variants (50-60%) of the species might produce pigments.
4. P agar
- Smooth, glistening, and usually opaque colonies are seen which are raised to slightly convex, circular, usually entire, and 4.0 to 9.0 mm in diameter.
- Colony pigment is variable; however, most strains are not pigmented or have a slight yellow tint which increased in intensity with age.
5. Thioglycolate medium
- Growth occurs in both the aerobic and anaerobic portions of the thioglycolate medium.
- The growth in the anaerobic portion is usually dense or shows a gradient from dense to light growth in the deeper, more anaerobic, portion of the medium.
Biochemical characteristics of Staphylococcus saprophyticus
The biochemical characteristics of S. saprophyticus can be tabulated as follows:
| S.N | Biochemical Characteristics | S. saprophyticus |
| 1. | Capsule | Most of the strains are capsulated. |
| 2. | Shape | Cocci |
| 3. | Catalase | Positive (+) |
| 4. | Oxidase | Negative (-) |
| 5. | Citrate | Negative (-) |
| 6. | Methyl Red (MR) | Negative (-) |
| 7. | Voges Proskauer (VR) | Negative (-) |
| 8. | Urease | Positive (+) |
| 9. | Coagulase | Negative (-) |
| 10. | Gas | Positive (+) |
| 11. | H2S | Positive (+) |
| 12. | Hemolysis | Negative (-) |
| 13. | Motility | Negative (-) |
| 14. | Nitrate Reduction | Negative (-) |
| 15. | Gelatin Hydrolysis | Negative (-) |
| 16. | Pigment Production | Negative (-) |
| 17. | Novobiocin resistantce | Resistant |
| 18. | Bile esculin test | Negative (-) |
Fermentation
| S.N | Substrate | S. saprophyticus |
| 1. | Mannitol | Positive (+) |
| 2. | Glucose | Positive (+) It ferments glucose mainly to ethanol, acetic acid, and CO2, and only small amounts of lactic and formic acid are produced. |
| 3. | Fructose | Positive (+) |
| 4. | Galactose | Positive (+) |
| 5. | Lactose | Positive (+) |
| 6. | Maltose | Positive (+) |
| 7. | Mannose | Negative (-) |
| 8. | Raffinose | Negative (-) |
| 9. | Ribose | Variable |
| 10. | Sucrose | Positive (+) |
| 11. | Starch | Negative (-) |
| 12. | Trehalose | Positive (+) |
| 13. | Xylose | Negative (-) |
| 14. | Salicin | Negative (-) |
| 15. | Glycerol | Positive (+) |
| 16. | DNase | Negative (-) |
| 17. | Dulcitol | Negative (-) |
| 18. | Cellobiose | Negative (-) |
| 19. | Rhamnose | Negative (-) |
| 20. | Arabinose | Negative (-) |
| 21. | Inulin | Negative (-) |
| 22. | Sorbitol | Negative (-) |
| 23. | Pyruvate | Negative (-) |
Enzymatic Reactions
| S.N | Enzymes | S. saprophyticus |
| 1. | Hyaluronidase | Variable |
| 2. | Acetoin | Positive (+) |
| 3. | Phosphatase | Negative (-) |
| 4. | Ornithine Decarboxylase | Variable |
| 5. | D-serine Deaminase | Positive (+) |
| 6. | Arginine Dehydrolase | Negative (-) |
Various amino acids like arginine, cystine, glycine, leucine, proline, and valine are essential for the growth of S. saprophyticus.
Virulence factors of Staphylococcus saprophyticus
S. saprophyticus is considered responsible for about 20-40% of all UTIs observed in young and middle-aged women. Up to date, several virulence factors have already been described in S. saprophyticus, including some surface proteins. Besides, some strains of S. saprophyticus have the ability to create biofilms, increasing their virulence, especially in patients with catheters.
Some of the virulence factors associated with infections caused by S. saprophyticus are:
1. Surface proteins
- Six different surface proteins are associated with the ability of S. saprophyticus to cause infections.
- Among them, two non-covalently surface-associated proteins are characterized so far. One of which is a protein associated with the surface of S. saprophyticus (Ssp) and another is the fibronectin-binding autolysin (Aas).
- Besides, four-cell wall-anchored proteins; the uro-adherence factor A (UafA), the collagen-binding serine-aspartate-repeat protein (SdrI), the uro-adherence factor B (UafB), and a surface protein F of S. saprophyticus have also been identified that aid in the attachment and colonization of the urogenital epithelium.
- These surface proteins result in hemagglutination, causing the bacteria to bind to fibronectin and human ureters as a result of their autolytic and adhesive properties.
2. Enzymes
- S. saprophyticus possess the enzyme urease that leads to alkalization of the urine and thus may result in the formation of kidney and bladder stones.
- Besides, urease is also associated with the formation of urinary calculi that aids in the inflammation in the bladder and the dissemination of the bacteria to other organs.
- D-serine deaminase is another enzyme that supports the survival of the organism in the urinary tract.
- It is essential for the survival of the uropathogens in the urinary environment as it confers the tolerance of the organism to the toxic concentration of D-serine in the urine.
- The surface-associated lipase present on the cell wall forms fimbria-like surface appendages, helping the bacteria to maintain tight adherence to these surfaces.
3. Biofilm formation
- Some strains of S. saprophyticus are capable of forming biofilms around medical devices like catheters that might result in nosocomial or hospital-acquired infections.
- Biofilm formation provides protection to the bacteria as it works as a barrier against the immune cells as well as antimicrobial agents.
- The process of biofilm formation is assisted by the presence of fibrinogen-binding adhesions and several other enzymes.
Pathogenesis of Staphylococcus saprophyticus
About 20-40% of all UTIs occurring in middle-aged women are found to be caused due to S. saprophyticus. These infections are also common in women within a few days after having sex, which is why these infections are termed as ‘honeymoon cystitis’. Besides, complications like acute pyelonephritis, urethritis, epididymitis, and prostatitis are also found to be caused by S. saprophyticus.
The pathogenesis of S. saprophyticus during urinary infection can be explained as follows:
1. Adhesion/ Attachment/ Colonization
- The gastrointestinal tract is found to be a major reservoir of S. saprophyticus which is assumed to be the route of entry to the urinary tract.
- Bacterial colonization of the bladder and ureter epithelium by S. Saphrophyticus occurs via several different types of adhesions.
- Various virulence factors like surface-proteins act as hemagglutinin and hemolysin and bind to the fibrinogen present on the surface of the urogenital cells.
- Other adhesins and wall-associated autolysins along with the enzyme lipase help maintain a tight adherence to the cell walls.
- The production of extracellular slime also prevents the attacks of immune cells to the colonizing bacteria.
2. Invasion
- Colonization of the urinary tract is the primary mode of infection by S. saprophyticus, but in some cases, invasive activities are seen where the infection ultimately leads to bacteremia.
- The enzyme urease helps to maintain the environment required for the growth of the organism while causing the formation of stones or calculi throughout the urinary tract.
- It is assumed that the urinary tract obstruction caused by a renal stone enables S. saprophyticus to reach the renal pelvis easily, and aggravate the tissue invasiveness of bacteria from the urinary tract.
- Although S. saprophyticus bacteremia from UTI is rare, obstructive nephrolithiasis is considered to be a predisposing factor for bacteremia from UTI.
Catheters-related infections
- In the case of catheter-related infection, the pathogenesis of the infection can be explained due to biofilm formation.
- The biofilm formation is supported by various cell-wall associated adhesions that adhere the bacteria to the abiotic surfaces.
- The accumulation of bacteria is then brought about by the production of the extracellular matrix or slime.
- The biofilm provides protection against the innate immune system and the antimicrobial agents, thus promoting the growth and colonization by the organism.
Clinical manifestations of Staphylococcus saprophyticus
- The most common and important clinical manifestation of S. saprophyticus infection is urinary tract infection.
- Common symptoms include inflammation of the lower tract, such as hematuria and pyuria, are observed.
- Besides, other common symptoms include dysuria, urinary frequency, urinary urgency, and suprapubic pain.
- Even though the infections can be treated readily via drug administration, if left untreated, they may progress to pyelonephritis, and untreated pyelonephritis may lead to further complications, such as renal insufficiency.
- The patients with pyelonephritis might also experience back or flank pain, nausea, and fever or chills
- In case of severe infections, bacteremia might be seen with might even lead to sepsis. Other severe complications like acute pyelonephritis, urethritis, epididymitis, and prostatitis are also common in some patients.
Lab Diagnosis of Staphylococcus saprophyticus
As with most bacterial infections, the collection of clinical specimens is the first step of laboratory diagnosis. In the case of S. saprophyticus, urine is the clinical specimen. However, in some cases, stool and blood samples should also be collected. Diagnosis of the organism can be achieved via morphological and biochemical identification of the organism along with various molecular tests.
The methods of lab diagnosis for infections caused by S. saprophyticus are:
1. Morphological characteristics and Biochemical tests
- Direct microscopic examination of these specimens may provide a rapid, presumptive report of gram-positive cocci resembling staphylococci.
- Direct observation is followed by isolation of the organism from primary clinical specimens on selective culture media like Mannitol salt agar or blood agar supplemented with 5 per cent sheep blood), following an incubation period of 18–24 h in the air at 35–37°C.
- Initial identification can be made by observing the colonies on the culture media. The isolated colonies can then be subjected to various biochemical tests.
- S. saprophyticus can be distinguished from other staphylococci primarily on the basis of cell wall composition, carbohydrate reaction pattern, and, to a lesser extent, on low acid production under anaerobic conditions, production of acetylmethylcarbinol, the failure to reduce nitrate, and resistance to novobiocin.
2. Rapid Identification kits
- Several rapid identification kits and automated systems can be found that are capable of identification of most species and subspecies within a few hours to one day with an accuracy of 70–90 percent.
- These kits are based on various factors like antibiotic susceptibility, enzyme production, immunological reactions, and cellular fatty acid analysis.
3. Molecular diagnosis
- Molecular methods usually include tests that help in the identification of the organism at a molecular level.
- One of the essential molecular methods is Polymerase Chain Reaction (PCR) which helps in the amplification and detection of bacterial DNA.
- Besides, DNA sequencing can also be done to determine the DNA sequence of the bacteria that can then be used for its identification.
- Another essential and successful diagnostic method is the analysis of ribosomal RNA by the application of restriction fragment length polymorphisms (RFLP) methods.
Treatment of Staphylococcus saprophyticus infection
- Treatment with outpatient antibiotics is indicated in symptomatic or complicated UTIs and pyelonephritis.
- The specific local resistant patterns are to be considered while choosing the appropriate antibiotic drug.
- The drug of choice in the case of uncomplicated S. saprophyticus UTIs is nitrofurantoin (Macrobid) which is to be taken 100 mg orally twice daily for five days or seven days in complicated cases.
- Trimethoprim-sulfamethoxazole (TMP-SMX) is another drug that is administered at a dosage of 160 mg/800 mg by mouth twice daily for three days given alternatively in uncomplicated cases.
- Symptomatic treatment for pain and nausea should also be addressed, and in some cases, acute uncomplicated UTIs are unlikely to cause renal injury, which should also be treated.
Prevention of Staphylococcus saprophyticus infection
- Because S. saprophyticus is mostly known to cause urinary tract infections, preventive methods for urinary tract infections can be applied to prevent the infections caused by S. saprophyticus.
- Healthy sanitation practices are critical to prevent all kinds of urinary tract infections.
- Taking enough water throughout the day also helps minimize the chances of urinary tract infections.
- Because S. saprophyticus is associated with infections caused soon after sexual intercourse, it is also advised to empty the bladder soon after.
References
- Topley WWC (2007). Topley and Wison’s Microbiology and Microbial Interactions; Bacteriology, 2 Vol. Tenth Edition. John Wiley and Sons Ltd.
- Raul Raz, Raul Colodner, Calvin M. Kunin, Who Are You—Staphylococcus saprophyticus?, Clinical Infectious Diseases, Volume 40, Issue 6, 15 March 2005, Pages 896–898, https://doi.org/10.1086/428353
- Gatermann S, John J, Marre R. Staphylococcus saprophyticus urease: characterization and contribution to uropathogenicity in unobstructed urinary tract infection of rats. Infect Immun. 1989;57(1):110-116. doi:10.1128/IAI.57.1.110-116.1989
- Makoto Kuroda, Atsushi Yamashita, Hideki Hirakawa (2005). Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. National Academy of Sciences Sep 2005, 102 (37) 13272-13277; DOI: 10.1073/pnas.0502950102
- KARL H. SCHLEIFER AND WESLEY E. KLOOS (1975). Isolation and Characterization of Staphylococci from Human Skin. INTERNATIONAL JOURNAL OF SYSTEMATIC BACTERIOLOGY Jan. 1975, p. 50-61.
- Weslley de Paiva-Santos, Viviane Santos de Sousa, Marcia Giambiagi-deMarval, Occurrence of virulence-associated genes among Staphylococcus saprophyticus isolated from different sources, Microbial Pathogenesis, Volume 119, 2018, Pages 9-11, ISSN 0882-4010, https://doi.org/10.1016/j.micpath.2018.03.054.
- King, N.P., Sakinç, T., Ben Zakour, N.L. et al. Characterization of a cell wall-anchored protein of Staphylococcus saprophyticus associated with linoleic acid resistance. BMC Microbiol 12, 8 (2012). https://doi.org/10.1186/1471-2180-12-8
- Hedman P, Ringertz O, Eriksson B, et al. Staphylococcus saprophyticus found to be a common contaminant of food. J Infect. 1990;21(1):11-19. doi:10.1016/0163-4453(90)90554-l
- Hur J, Lee A, Hong J, et al. Staphylococcus saprophyticus bacteremia originating from Urinary Tract Infections: A Case Report and Literature Review. Infect Chemother. 2016;48(2):136-139. doi:10.3947/ic.2016.48.2.136
- Pead L, Maskell R, Morris J. Staphylococcus saprophyticus as a urinary pathogen: a six year prospective survey. Br Med J (Clin Res Ed). 1985;291(6503):1157-1159. doi:10.1136/bmj.291.6503.1157
- Ehlers S, Merrill SA. Staphylococcus Saprophyticus. [Updated 2020 Jun 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482367/
- Viviane Santos de Sousa, Ana Paula de Souza da-Silva, Nunes Botelho, Renata Cristina Picão, Sérgio Eduardo Longo Fracalanzza, Lee Woodland Riley, George Sensabaugh, Beatriz Meurer Moreira, “Staphylococcus saprophyticus Recovered from Humans, Food, and Recreational Waters in Rio de Janeiro, Brazil”, International Journal of Microbiology, vol. 2017, Article ID 4287547, 11 pages, 2017. https://doi.org/10.1155/2017/4287547
- Micael Widerström, Johan Wiström, Sven Ferry, Carina Karlsson, Tor Monsen (2007). Molecular Epidemiology of Staphylococcus saprophyticus Isolated from Women with Uncomplicated Community-Acquired Urinary Tract Infection. Journal of Clinical Microbiology May 2007, 45 (5) 1561-1564; DOI: 10.1128/JCM.02071-06
- RUPP et al (1992). Colonization of the Female Genital Tract with Staphylococcus saprophyticus. JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 1992, P. 2975-2979
- Tridevi et al (2015). Antimicrobial Sensitivity, Biochemical Characteristics and Biotyping of Staphylococcus saprophyticus: An Impact of Biofield Energy Treatment. J Women’s Health Care 2015, 4:6 DOI: 10.4172/2167-0420.1000271
Sources
- 7% – https://microbenotes.com/staphylococcus-epidermidis/
- 3% – https://www.sciencedirect.com/science/article/pii/S0882401017313396
- 2% – https://www.icjournal.org/DOIx.php?id=10.3947/ic.2016.48.2.136
- 2% – https://pubmed.ncbi.nlm.nih.gov/29493989/
- 2% – https://microbenotes.com/staphylococcus-lugdunensis/
- 2% – https://microbenotes.com/staphylococcus-hominis/
- 1% – https://www.theinternatwork.com/nephrology-week/2020/4/1/day-5-uti
- 1% – https://www.statpearls.com/articlelibrary/viewarticle/29454/
- 1% – https://www.researchgate.net/publication/237391460_Isolation_and_Characterization_of_Staphylococci_from_Human_Skin_I_Amended_Descriptions_of_Staphylococcus_epidermidis_and_Staphylococcus_saprophyticus_and_Descriptions_of_Three_New_Species_Staphylococc
- 1% – https://microbenotes.com/streptococcus-bovis/
- 1% – https://europepmc.org/article/MED/29493989
- 1% – http://www.thistle.co.za/pdf_files/education/microbiology/microbiology_legends/Cycle_40/Cycle%2040%20Organism%201%20-%20S.%20saprophyticus.pdf
- <1% – https://www.verywellhealth.com/acute-low-back-pain-296544
- <1% – https://www.thomassci.com/scientific-supplies/Mannitol-Salt-Agar
- <1% – https://www.sciencedirect.com/topics/neuroscience/trimethoprim-sulfamethoxazole
- <1% – https://www.sciencedirect.com/topics/immunology-and-microbiology/pyelonephritis
- <1% – https://www.sciencedirect.com/science/article/pii/S0960982206024912
- <1% – https://www.researchgate.net/publication/7997903_Who_Are_You–Staphylococcus_saprophyticus
- <1% – https://www.researchgate.net/publication/5754808_Influence_of_environmental_conditions_on_biofilm_formation_by_Hafnia_alvei_strains
- <1% – https://www.researchgate.net/publication/38014194_SdrI_of_Staphylococcus_saprophyticus_is_a_multifunctional_protein_Localization_of_the_fibronectin-binding_site
- <1% – https://www.researchgate.net/publication/315832556_UK_Standards_for_Microbiology_Investigations-standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical-laboratories_UK_Standards_for_Microbiology_Investigations_are_produced_in
- <1% – https://www.neb.com/applications/cloning-and-synthetic-biology/dna-analysis/dna-sequencing
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4582588/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2764210/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620709/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1865898/
- <1% – https://www.jstor.org/stable/4453343
- <1% – https://www.drugs.com/macrobid.html
- <1% – https://www.coursehero.com/file/40333444/S-saprophyticuspptx/
- <1% – https://www.cosmopolitan.com/sex-love/a28172266/sore-vagina-after-sex/
- <1% – https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)62792-0/fulltext
- <1% – https://stanfordhealthcare.org/medical-conditions/womens-health/urinary-tract-infection/types.html
- <1% – https://quizlet.com/282714399/microbial-growth-media-flash-cards/
- <1% – https://pubmed.ncbi.nlm.nih.gov/6377440/
- <1% – https://pubmed.ncbi.nlm.nih.gov/2475423/
- <1% – https://microbiologyinpictures.com/staphylococcus%20aureus.html
- <1% – https://iai.asm.org/content/iai/57/1/110.full.pdf
- <1% – https://europepmc.org/articles/PMC1201578
- <1% – https://brainly.in/question/6245929
- <1% – https://biologydictionary.net/peptidoglycan/
- <1% – http://www.ufrgs.br/imunovet/molecular_immunology/cellculture.html
- <1% – http://www.columbia.edu/itc/hs/medical/pathophys/id/2008/utiNotes.pdf
- <1% – http://www.cir-safety.org/sites/default/files/amacid092012rep.pdf
- <1% – http://vanat.cvm.umn.edu/ungDissect/Lab15/Lab15.html

Why is the H2S positive when the media used to test for H2S (SIM) is used as a differential media for enterobacteria since the Indole and motility are negative shouldn’t the H2S be negative also?