Interesting Science Videos
What is Staphylococcus lugdunensis?
Staphylococcus lugdunensis is a Gram-positive, coagulase-negative coccus that is a part of human normal flora but recently has been associated with various skin and soft tissue infections.
- S. lugdunensis has a significant pathogenic potential compared with other coagulase-negative staphylococci owing to its various virulence factors.
- Like all Staphylococci, S. lugdunensis is also clustering Gram-positive cocci, non-motile, non-spore-forming, and facultatively anaerobic.
- It was first isolated and described by Jean Freney et al. in 1988 from human clinical specimens like blood, lymph nodes, abscess, and thoracic drain.
- S. lugdunensis is named after Lugdunum, the Latin name of Lyon, a French city where the organism was first isolated.
- It causes various superficial infections that are close to the infections caused by S. aureus along with bone and joint infections and native valve endocarditis in rare cases.
- S. lugdunensis can be distinguished from S. aureus in that it doesn’t have the enzyme coagulase and can be differentiated from other coagulase-negative species because of its susceptibility to most antibiotics.
- S. lugdunensis is an integral part of the normal skin flora, primarily of the lower abdomen, extremities, groin, perineal areas, and the nail bed of the first toe. Besides, it is also rarely isolated from the face and nares.
- Although a commensal, S. lugdunensis has the ability to cause aggressive infections, resembling those of S. aureus rather than of other CoNS.
- This organism had the potential to be an opportunistic pathogen and thus is considered an unusually virulent CoNS that can cause many types of infection, ranging from superficial skin infections to life-threatening endocarditis.
Classification of Staphylococcus lugdunensis
- Classification of different species of the genus Staphylococci is based on various factors ranging from morphology, chemical properties, amino acid sequences, biochemical characteristics, and nucleotide sequences.
- Staphylococcus spp. are primarily classified on the basis of DNA–DNA hybridization where members of the same species demonstrate relative DNA-binding values of generally 70 percent or greater.
- The primary basis for the classification of the S. lugdunensis species is the base composition of the DNA, which was found to be 32 mol% G+C.
| Domain: | Bacteria |
| Phylum: | Firmicutes |
| Class: | Bacilli |
| Order: | Bacillales |
| Family: | Staphylococcaceae |
| Genus: | Staphylococcus |
| Species: | S. lugdunensis |
Habitat of Staphylococcus lugdunensis
- Human is the primary host of S. lugdunensis, and there is no temporary or intermediate host.
- It is a part of the human normal flora present on the skin throughout the body but is mostly concentrated in areas with higher humidity and thinner skin layers.
- S. lugdunensis is primary located in the areas around the lower abdomen, groin, and perineal areas. Besides, it is found in large numbers in the nail bed of the first toe.
- It also forms a part of the normal flora of the nares and nasal cavity.
- Because it is mostly found in the lower areas of the body, it is also termed as the ‘below the belt’s colonizer.
- Areas that are relatively dry to moderately moist and bathed in an emulsion of lipids and eccrine sweat containing lactic acid–lactate, amino acids, urea, and electrolytes are considered excellent habitats for S. lugdunensis.
Morphology of Staphylococcus lugdunensis
- S. lugdunensis is a Gram-positive coccus with an average diameter of 0.8–1.0 μm. These occur mostly either singly or form pairs, clusters, and chains composed of three to five cells, that divide in more than one plane to form irregular grapelike clusters.
- It is non-motile, non-spore-forming, facultatively anaerobic, and usually unencapsulated or limited capsule formation.
- The cell wall of S. lugdunensis contains peptidoglycan and teichoic acid, and the diamino acid present in the peptidoglycan is L-lysine.
- The cell membrane is typical of all Staphylococci with lipid-protein bilayer composed mainly of phospholipids and different proteins.
- Phospholipids, glycolipids, menaquinones, and carotenoids make up the major lipid components of the membrane, whereas there are different proteins with different functions.
- Like all other coagulase-negative Staphylococci, S. lugdunensis also has fewer cell wall adhesions and cell-wall associated proteins.
- S. lugdunensis have specific cell-wall adhesins like SdrF and SdrG that act as fibrinogen binding adhesion molecules that aids in the attachment and colonization by the organism.
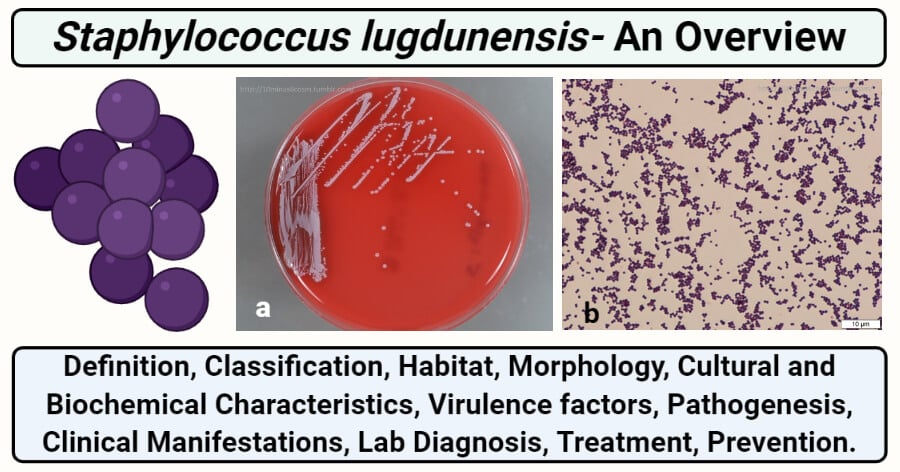
Figure: a- Colonies look grayish, but appear a bit less pigmented compared with S. aureus. b- Gram stain showing Gram-positive cocci in clusters. Image Source: 10minus6cosm.
Cultural Characteristics of Staphylococcus lugdunensis
Staphylococci from clinical specimens are usually isolated in primary culture on blood agar and in a fluid medium such as thioglycolate broth. Besides, other selective media like Mannitol Salt Agar, Baird-Parker agar, Tellurite Polymyxin egg yolk agar, and P agar can also be used for enrichment and isolation. Cultural characteristics of the organism can be used for the primary identification of the organism during lab diagnosis. The temperature range for good growth is 30–45°C, whereas weak growth is observed at 20°C. The organism can tolerate 10% NaCl, but delayed growth can be seen on 15% NaCl.
1. Nutrient Agar (NA)
- Circular, cream-colored to white colonies of S. lugdunensis is observed on NA. The colonies are mostly 1 mm in diameter with an entire margin.
- The colonies have raised elevation and a dense center with transparent borders.
2. Mannitol Salt Agar (MSA)
- Small pink to red colonies are formed on MSA. The media remains red as the bacterium cannot ferment mannitol.
- The colonies are 1-2 mm in diameter with an entire margin.
3. P agar
- Colonies are cream or pale yellow to golden color that is glistening, smooth with an entire margin.
- The colony morphology might be somewhat variable. The colony diameter is mostly 1–4 mm after incubation for 72 h at 35°C on P agar.
4. Blood Agar (BA)
- Wrinkled, medium-sized (1-4 mm in diameter), beta-hemolytic, opaque, rough white colonies are observed. Colony pleiomorphism is common on blood agar.
- Prominent β-hemolysis is seen after about two days of incubation.
5. Thioglycollate medium
- Abundant anaerobic growth is seen with an overnight incubation at 35-37°C.
Biochemical Characteristics of Staphylococcus lugdunensis
The biochemical characteristics of S. lugdunensis can be tabulated as follows:
| S.N | Biochemical Characteristics | S. lugdunensis |
| 1. | Capsule | Limited capsule formation |
| 2. | Shape | Cocci |
| 3. | Catalase | Positive (+) |
| 4. | Oxidase | Negative (-) |
| 5. | Citrate | Negative (-) |
| 6. | Methyl Red (MR) | Negative (-) |
| 7. | Voges Proskauer (VR) | Negative (-) |
| 8. | Urease | Positive (+) |
| 9. | Coagulase | Negative (-) |
| 10. | DNase | Negative (-) |
| 11. | Clumping factor | Positive (+) |
| 12. | Gas | Positive (+) |
| 11. | H2S | Positive (+) |
| 12. | Hemolysis | Β-hemolytic |
| 13. | Motility | Negative (-) |
| 14. | Nitrate Reduction | Positive (+) |
| 15. | Gelatin Hydrolysis | Negative (-) |
| 16. | Pigment Production | Variable |
| 17. | Novobiocin resistantce | Susceptible |
| 18. | Bile esculin test | Negative (-) |
Fermentation
| S.N | Substrate | S. lugdunensis |
| 1. | Mannitol | Negative (-) |
| 2. | Glucose | Positive (+) |
| 3. | Fructose | Positive (+) |
| 4. | Galactose | Positive (+) |
| 5. | Lactose | Positive (+) |
| 6. | Maltose | Positive (+) |
| 7. | Mannose | Positive (+) |
| 8. | Raffinose | Negative (-) |
| 9. | Ribose | Negative (-) |
| 10. | Sucrose | Positive (+) |
| 11. | Starch | Negative (-) |
| 12. | Trehalose | Positive (+) |
| 13. | Xylose | Negative (-) |
| 14. | Salicin | Positive (-) |
| 15. | Glycerol | Positive (+) |
| 16. | Dulcitol | Negative (-) |
| 17. | Cellobiose | Negative (-) |
| 18. | Rhamnose | Negative (-) |
| 19. | Arabinose | Negative (-) |
| 20. | Inulin | Negative (-) |
| 21. | Sorbitol | Negative (-) |
| 22. | Pyruvate | Negative (-) |
Enzymatic Reactions
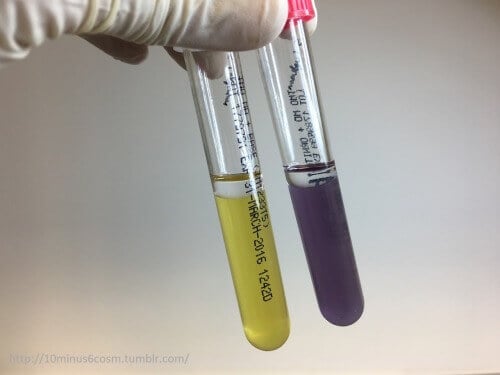
Figure: S. lugdunensis is positive for the ornithine decarboxylase test. The left tube is the control (no amino acid), the right contains ornithine. Both tubes contain the indicator Bromocresol purple which turns from yellow (acid pH) to purple (alkaline pH) when the test is positive. Image Source: 10minus6cosm.
| S.N | Enzymes | S. lugdunensis |
| 1. | Hyaluronidase | Variable |
| 2. | Acetoin | Positive (+) |
| 3. | Phosphatase | Negative (-) |
| 4. | Ornithine Decarboxylase | Positive (+) |
| 5. | Pyrrolidonyl aminopeptidase | Positive (+) |
| 6. | β-galactosidase | Negative (-) |
Virulence Factors of Staphylococcus lugdunensis
Staphylococcus lugdunensis has emerged lately as a significant human pathogen with notable clinical and microbiological characteristics that stand out among other coagulase-negative staphylococci. Biofilm formation is a predominant virulence mechanism employed by S. lugdunensis, whose genome harbors homologs of the ica operon that encode the proteinaceous biofilm extracellular matrix. Besides, the organism has the potential to interact with host tissues and proteins that may coat foreign surfaces during implantation of the medical devices. Different surface adhesins also aid in the binding potential of the organism which supports both colonization and biofilm formation.
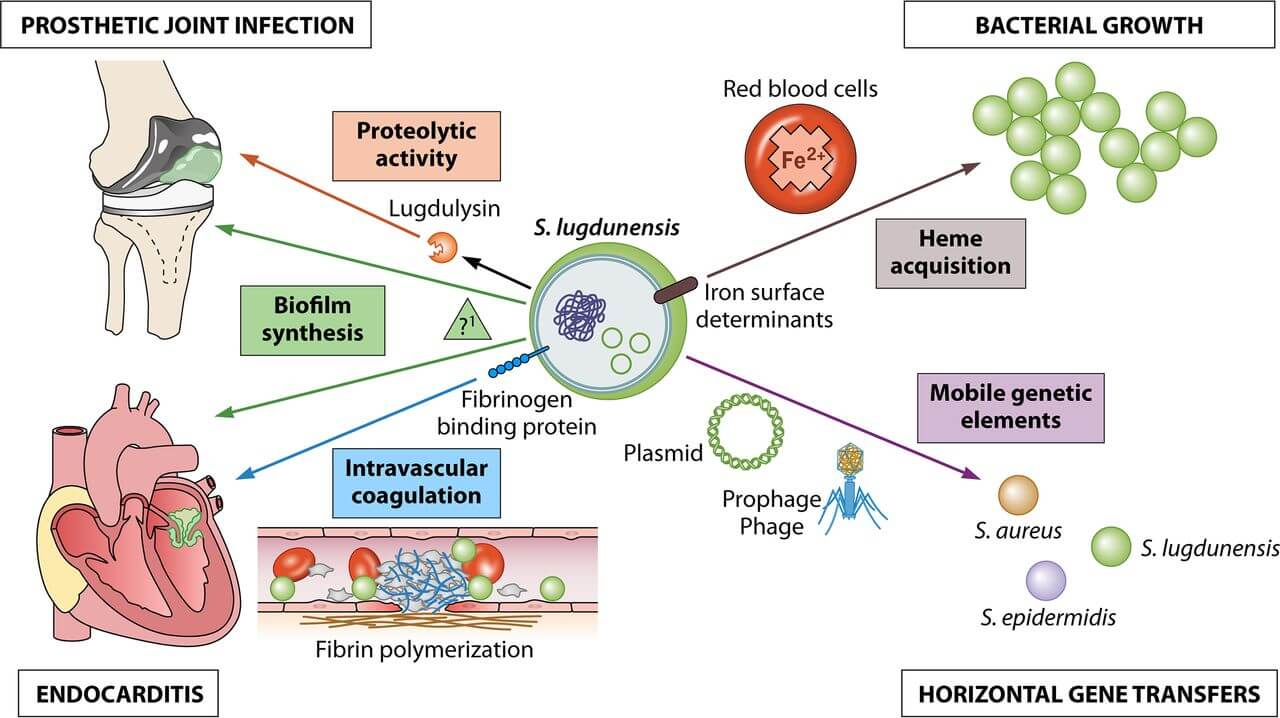
Figure: Clinical and bacteriological roles of the main putative virulence factors identified in S. lugdunensis. Image Source: American Society for Microbiology.
1. Biofilm formation
- One of the major factors in the pathogenesis of staphylococcal infections is biofilm formation.
- Biofilm is macroscopic aggregates of microorganisms enclosed in an extracellular matrix either produced by the organism or derived from the environment.
- Biofilm development allows for the deep-sited cells to become more resistant to administered antibiotics and the body’s natural mechanisms, interfering with attempts by the host immune system to clear the infection.
- Biofilm formation in S. lugdunensis is different from that in S. aureus or S. epidermidis, in that it is proteinaceous and is also enhanced by the presence of a foreign body like a medical implant device.
- It occurs in a two-step process; the first step involving the binding and colonization of the surface by the bacteria and the step involving the accumulation of bacteria and release of the extracellular matrix.
- The atlL gene present in the organism is responsible for the production of an autolysin involved in cell separation, stress-induced autolysis, and contributes to bacterial pathogenesis.
- This autolysin confers initial bacterial attachment and release of extracellular DNA which are two important processes during biofilm formation.
- The attachment is further supported by adherence of bacteria to surfaces which is promoted by surface protein adhesins such as the fibrinogen-binding clumping factor A or fibronectin-binding proteins.
- This process is followed by proliferation, accumulation, and intercellular interactions mediated by the icaADBC-encoded Polysaccharide Intercellular Adhesins (PIA) or surface proteins such as Bap, SasG, SasC, protein A, or fibronectin-binding proteins (FnBPs).
- Besides, another gene isd is also found to support biofilm formation as it recognizes and binds several host proteins and can confer resistance to skin fatty acids.
2. Proteins and adhesins
- A protein has been identified in S. lugdunensis that specifically binds von Willebrand factor (vWf) that is a blood plasma glycoprotein produced by endothelial cells and platelets involved in coagulation, via binding to platelets and subendothelial collagen after vascular injury.
- This protein has organizational similarity to clumping factor A of S. aureus and also supports the binding and clumping of blood.
- S. lugdunensis isolates also possess the fbl gene, which encodes a surface-located fibrinogen-binding adhesin, referred to as the Fbl protein that mediates binding to the fibrinogen γ-chain.
- S. lugdunensis also possess the slush locus, which encodes for hemolytic peptides with delta-toxin-like activity.
Pathogenesis of Staphylococcus lugdunensis
Even though S. lugdunensis is present on the human body as a commensal, it is known to act as an opportunistic pathogen and cause several infections of different organs.
The pathogenesis of S. lugdunensis can be explained in the following ways:
1. Attachment/ Adhesion/ Colonization
- The first step during the infection by S. lugdunensis is the attachment of the organism to the skin surface.
- The attachment of the bacterium is brought about by a number of factors like adhesins and proteins that together allow the bacteria to specifically bind to certain proteins on the surface of the skin.
- The factors involved in the attachment of the bacteria include surface protein adhesins such as the fibrinogen-binding clumping factor A or fibronectin-binding proteins.
- In the context of medical devices, the surface of the device becomes coated with host-derived plasma proteins, extracellular matrix proteins, and coagulation products (platelets and thrombin) immediately the following adhesion.
- Autolysin produced by the atlL gene present in the organism is involved in cell separation, stress-induced autolysis which contributes to the process of biofilm formation.
- In the case of medical devices, wall teichoic acid enhances the initial adhesion of S. lugdunensis to medical devices by binding to adsorbed fibronectin.
- In cases with artificial heart valves, the bacteria attach to the surface of the valve, which further increases the rate of biofilm formation.
2. Biofilm Formation
- Biofilm formation is the most important virulence factor during the pathogenesis of infections caused by S. lugdunensis.
- The attachment of the bacteria to the biotic or abiotic surface is the first step of biofilm formation. Once the attachment is established, bacteria accumulate themselves to form a solid film.
- The proliferation, accumulation, and intercellular interactions are mediated by the icaADBC-encoded Polysaccharide Intercellular Adhesins (PIA) or surface proteins such as Bap, SasG, SasC, protein A, or fibronectin-binding proteins (FnBPs).
- These proteins allow the bacteria to bind with one another, which results in the formation of a film that protects the bacteria present underneath from the immune cells as well as the molecules of antimicrobial agents.
- The biofilm of S. lugdunensis is more proteinaceous than the one formed by S. epidermidis as it is made up of accumulation associated protein (Aap).
3. Dispersal
- Once the biofilm is constructed, the bacterial autolysin encoded by the gene atlL results in cell segregation and release of extracellular DNA.
- The separated bacteria can then move through the bloodstream and reach various organs and even result in sepsis or toxic shock syndrome.
Clinical Manifestations of Staphylococcus lugdunensis
S. lugdunensis have now emerged as pathogenic bacteria, found to be involved in severe infections, particularly, osteoarticular infections, foreign-body-associated infections, bacteremia, and endocarditis. Besides, some instances of joint and bone infections, peritonitis, oral and ocular infections caused by S. lugdunensis have also been recorded.
Different infections that are found to be associated with S. lugdunensis are given below:
1. Endocarditis
- Endocarditis is one of the most harmful infections caused by S. lugdunensis with a very high mortality rate (70%).
- This infection is mostly associated with artificial heart valves that are mostly hospital-acquired and occur in immune-compromised individuals.
- It is often accompanied by an underlying condition, like fever chills, and might cause fatigue and aching joints and muscles.
- Endocarditis most often than not requires the removal or replacement of the valve.
2. Skin and soft tissue infections
- Skin and soft tissue infections account for a prominent number of the total infections caused by S. lugdunensis.
- S. lugdunensis causes suppurative lesions, including furuncles, felons, and sebaceous cysts, at a higher frequency than other coagulase-negative staphylococci like S. epidermidis.
- Many S. lugdunensis skin infections, particularly abscesses, are localized in the perineal, inguinal, or pelvic girdle region.
3. Bloodstream infection and sepsis
- Bloodstream infections or sepsis is comparatively less frequent with S. lugdunensis infections as the bacteria is susceptible to most antibiotics and thus can be removed before a severe condition arises.
- Most of the bloodstream infections associated with this bacterium are catheter-associated and occurs in neonates.
- However, some instances of S. lugdunensis-induced septicemia and septic shock have been recorded in patients with recent surgeries.
Lab Diagnosis of Staphylococcus lugdunensis
As with most bacterial infections, the collection of clinical specimens is the first step of laboratory diagnosis. In the case of S. lugdunensis, clinical specimens like the scabs, joint aspirates, and pus aspirated from deep sites are to be collected. Diagnosis of disease in the case of S. epidermidis infections are mostly related to the identification of the organism.
1. Morphological and biochemical characteristics
- Direct microscopic examination of these specimens may provide a rapid, presumptive report of gram-positive cocci resembling staphylococci.
- Direct observation is followed by isolation of the organism from primary clinical specimens on selective culture media like blood agar supplemented with 5 percent sheep blood, following an incubation period of 18–24 h in the air at 35–37°C.
- Hemolysis of the medium is another method for the identification of the bacterium as it demonstrates β-hydrolysis.
- Initial identification can be made by observing the colonies on the culture media. The isolated colonies can then be subjected to various biochemical tests.
- The colonies and pigment production might be variable among the strains of S. lugdunensis, and thus the identification should be supplemented with biochemical tests.
- Depending on the microscopic observation, colony morphology, and biochemical tests, S. lugdunensis can be detected.
2. Commercial kits or automated systems
- Recently, many clinical laboratories have started to employ commercial identification kits or automated instruments that allow quick determination of bacterial species.
- In the case of S. lugdunensis, the identification is based on the analysis of their microbial cellular fatty acid compositions.
- Some of the common automated systems for the identification of S. lugdunensis include MicroScan Conventional Pos ID, Rapid Pos ID, and BBL Crystal Gram-Pos ID.
3. Molecular diagnosis
- Molecular methods of diagnosis include methods to differentiate microorganisms by unique nucleic acid sequences that are becoming more common in the clinical microbiology laboratories due to increasing technological advances, including real-time PCR and high-throughput DNA sequencing systems.
- The diversity in the sequence of 16S rRNA genes of staphylococci enables species-level identification.
- Thus, PCR amplification and sequencing of the 16S rRNA gene have become an option for molecular identification of pathogenic bacteria in diagnosis.
- Ribotyping, the analysis of rRNA by restriction fragment length polymorphism, is an alternative method for molecular differentiation of S. lugdunensis.
Treatment of Staphylococcus lugdunensis infections
- It is found that penicillin is a better option than oxacillin for treating S. lugdunensis infections.
- Because the organism is mostly susceptible to many available antibiotics, treatment of the infections is not a major issue.
- However, some infections like endocarditis and bloodstream infections might require removal of the medical implants and immediate treatments.
- Besides, other forms of treatments involving the hyperimmune serum from human donors or humanized monoclonal antibodies directed towards the surface components are also being studied.
Prevention of Staphylococcus lugdunensis infections
- Since infections associated with S. lugdunensis is often associated with nosocomial or hospital-acquired infections, regular cleaning and dressing wounds might work to avoid such infections.
- Also, maintaining proper hygiene and sanitation helps avoid various infections.
- Early diagnosis and treatment are imperative as it prevents the chances of bloodstream infections and severe cases.
References
- Topley WWC (2007). Topley and Wison’s Microbiology and Microbial Interactions; Bacteriology, 2 Vol. Tenth Edition. John Wiley and Sons Ltd.
- Bergey, D. H., Whitman, W. B., De, V. P., Garrity, G. M., & Jones, D. (2009). Bergey’s manual of systematic bacteriology: Vol. 3. New York: Springer.
- Bieber L, Kahlmeter G. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin Microbiol Infect. 2010;16(4):385-388. doi:10.1111/j.1469-0691.2009.02813.x
- Heldt Manica, L. A., & Cohen, P. R. (2017). Staphylococcus lugdunensis Infections of the Skin and Soft Tissue: A Case Series and Review. Dermatology and therapy, 7(4), 555–562. https://doi.org/10.1007/s13555-017-0202-5
- Taha, L., Stegger, M., & Söderquist, B. (2019). Staphylococcus lugdunensis: antimicrobial susceptibility and optimal treatment options. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology, 38(8), 1449–1455. https://doi.org/10.1007/s10096-019-03571-6
- Lebeurre, J., Dahyot, S., Diene, S., Paulay, A., Aubourg, M., Argemi, X., Giard, J. C., Tournier, I., François, P., & Pestel-Caron, M. (2019). Comparative Genome Analysis of Staphylococcus lugdunensis Shows Clonal Complex-Dependent Diversity of the Putative Virulence Factor, ess/Type VII Locus. Frontiers in microbiology, 10, 2479. https://doi.org/10.3389/fmicb.2019.02479
- Yazgi, H., & Uyanik, M. H. (2010). Atypical Colony Morphology of Staphylococcus lugdunensis Isolated from a Wound Specimen. The Eurasian journal of medicine, 42(1), 36–37. https://doi.org/10.5152/eajm.2010.10
- Sundqvist, M., Bieber, L., Smyth, R., & Kahlmeter, G. (2010). Detection and identification of Staphylococcus lugdunensis are not hampered by use of defibrinated horse blood in blood agar plates. Journal of clinical microbiology, 48(5), 1987–1988. https://doi.org/10.1128/JCM.02307-09
- Missineo, A., Di Poto, A., Geoghegan, J. A., Rindi, S., Heilbronner, S., Gianotti, V., Arciola, C. R., Foster, T. J., Speziale, P., & Pietrocola, G. (2014). IsdC from Staphylococcus lugdunensis induces biofilm formation under low-iron growth conditions. Infection and immunity, 82(6), 2448–2459. https://doi.org/10.1128/IAI.01542-14
- Xavier Argemi, Yves Hansmann, Philippe Riegel, Gilles Prévost. Is Staphylococcus lugdunensis Significant in Clinical Samples. Journal of Clinical Microbiology Oct 2017, 55 (11) 3167-3174; DOI: 10.1128/JCM.00846-17
- Kristi L. Frank, José Luis del Pozo, Robin Patel. From Clinical Microbiology to Infection Pathogenesis: How Daring To Be Different Works for Staphylococcus lugdunensis. Clinical Microbiology Reviews Jan 2008, 21 (1) 111-133; DOI: 10.1128/CMR.00036-07
- Giormezis N, Kolonitsiou F, Makri A, et al. Virulence factors among Staphylococcus lugdunensis are associated with infection sites and clonal spread. Eur J Clin Microbiol Infect Dis. 2015;34(4):773-778. doi:10.1007/s10096-014-2291-8
- van der Mee-Marquet, N., Achard, A., Mereghetti, L., Danton, A., Minier, M., & Quentin, R. (2003). Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. Journal of clinical microbiology, 41(4), 1404–1409. https://doi.org/10.1128/jcm.41.4.1404-1409.2003
- Taha, L., Stegger, M., & Söderquist, B. (2019). Staphylococcus lugdunensis: antimicrobial susceptibility and optimal treatment options. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology, 38(8), 1449–1455. https://doi.org/10.1007/s10096-019-03571-6
- Sidsel Böcher, Birgitte Tønning, Robert L. Skov, Jørgen Prag. Staphylococcus lugdunensis, a Common Cause of Skin and Soft Tissue Infections in the Community. Journal of Clinical Microbiology Apr 2009, 47 (4) 946-950; DOI: 10.1128/JCM.01024-08
- Freney, Jean; Brun, Yvonne; Bes, Michele; Meugnier, Helene; Grimont, Francine; Grimont, Patrick A.D.; Nervi, Chantal; Fleurette, Jean (April 1933). “Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., Two Species from Human Clinical Specimens” . International Journal of Systematic Bacteriology. 38 (2): 168–172. doi:10.1099/00207713-38-2-168

Information is kinda hard to understand with all the medical wording maybe a picture or two would help!