- The Shiga toxin-producing E. coli (STEC), is also alternatively referred to as verocytotoxin-producing E. coli (VTEC), and enterohemorrhagic E. coli (EHEC).
- All members of this group are defined by the presence of Shiga toxin 1 (Stx1) or 2 (Stx2). Some but not all EHEC strains are LEE positive and form A/E cytopathology, resembling EPEC strains.
- The most common serotype associated with human disease is O157:H7. However, it represents less than 50% of the responsible serotypes and the prevalent serotypes will vary geographically.
- It is prevalent mainly in industrialized countries (in contrast to other diarrheagenic E. coli which are common in developing regions).
- The infective dose of STEC is very low. Only a few organisms (<102 bacilli) are required to initiate the infection.
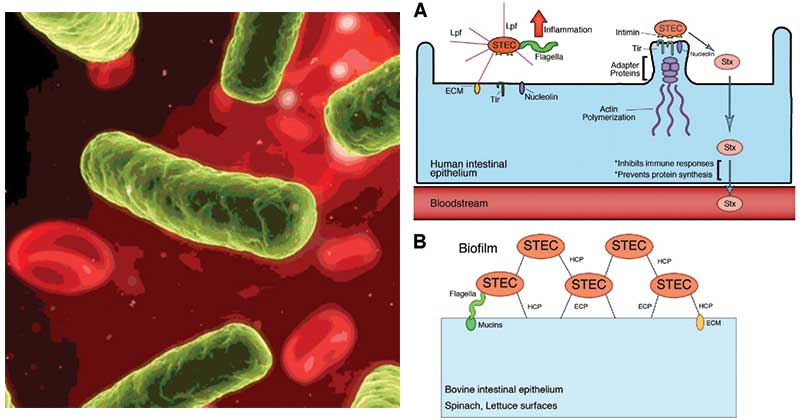
Image: Working colonization model for STEC infecting humans and persisting in ruminants. (A) The diagram shows a human intestinal epithelial cell displaying the first stages of colonization by STEC in the lumen. STEC O157 or non-O157 strains interact with the intestine via the Lpf fimbriae; this is followed by the formation of A/E lesions. Stx production occurs in the intestinal tract, and translocation across the intestinal lumen to the bloodstream results in its distribution to target organs, such as the kidneys. (B) STEC O157 and non-O157 strains are also capable of adhering and colonizing other surfaces, such as the bovine intestinal epithelium and the surfaces of different vegetables. Several adhesins, such as HCP, ECP, and flagella, have been associated with persistence in the bovine intestine and the formation of biofilms.
Image Source: Food Safety Magazine and DOI: 10.1128/IAI.05907-11
Interesting Science Videos
Disease Caused
Disease caused by STEC ranges from mild uncomplicated diarrhea to hemorrhagic colitis and Hemolytic uremic syndrome (HUS). Severe disease is more commonly associated with STEC O157:H7.
Risk Factors and the Mode of Transmission
- STEC disease is most common in the warm months, and the highest incidence is in children younger than 5 years.
- Most infections are attributed to the consumption of undercooked ground beef or other meat products, water, unpasteurized milk or fruit juices (e.g., cider made from apples contaminated with feces from cattle), uncooked vegetables such as spinach, and fruits.
- Ingestion of fewer than 100 bacteria can produce disease, and person-to-person spread occurs.
Toxins and Pathogenesis
- Stx1 is essentially identical to the Shiga toxin produced by Shigella dysenteriae (thus the source of the name); Stx2 has 60% homology.
- Both toxins are acquired by lysogenic bacteriophages.
- Both have one A subunit and five B subunits, with the B subunits binding to a specific glycolipid on the host cell (globotriaosylceramide [Gb3]).
- A high concentration of Gb3 receptors is found in the intestinal villi and renal endothelial cells.
- After the A subunit is internalized, it is cleaved into two molecules, and the A1 fragment binds to 28S rRNA and causes a cessation of protein synthesis.
- STEC strains with both Shiga toxins and attaching and effacing activity are more pathogenic than strains producing only one Shiga toxin.
- HUS has been preferentially associated with the production of Stx2, which has been shown to destroy glomerular endothelial cells.
- Damage to the endothelial cells leads to platelet activation and thrombin deposition which results in decreased glomerular filtration and acute renal failure.
- The Shiga toxins also stimulate the expression of inflammatory cytokines (e.g., tumor necrosis factor [TNF]-γ, interleukin [IL]- 6), enhancing expression of the B subunit receptor Gb3.
Clinical Features and Complications
- Disease caused by STEC ranges from mild uncomplicated diarrhea to hemorrhagic colitis with severe abdominal pain and bloody diarrhea.
- Initially, diarrhea with abdominal pain develops in patients after 3 to 4 days of incubation.
- Vomiting is observed in approximately half the patients, but a high fever is generally absent.
- Within 2 days of onset, the disease in 30% to 65% of patients progresses to bloody diarrhea with severe abdominal pain.
- Hemolytic uremic syndrome (HUS), a disorder characterized by acute renal failure, thrombocytopenia, and microangiopathic hemolytic anemia, is a complication in 5% to 10% of infected children younger than 10 years.
- Signs and symptoms of HUS can include:
- decreased urination
- swelling of limbs
- high blood pressure
- jaundice (yellowish discoloration of the skin and the whites of the eyes)
- seizures (fits) or other neurological symptoms
- bleeding into the skin.
Complications
Resolution of symptoms occurs in uncomplicated disease after 4 to 10 days in most untreated patients; however, death can occur in 3% to 5% of patients with HUS, and severe sequelae (e.g., renal impairment, hypertension, central nervous system [CNS] manifestations) can occur in as many as 30% of HUS patients.
Diagnosis
Sorbitol MacConkey agar: EHEC, in contrast to other E. coli, does not ferment sorbitol and produces pale colonies.
Rainbow agar: 0157 strains appear as black colonies on this medium as they are negative for beta-glucuronidase.
Toxin detection:
- Demonstration of cytotoxicity in Vero cell lines (gold standard method)
- Fecal toxin detection by ELISA or rapid tests
- PCR can be used 10 differentiate genes coding for Stxl and Stx2.
Treatment
- Complete resolution of symptoms typically occurs after 4 to 10 days in most untreated patients.
- In most cases, antibiotic therapy is not prescribed since antibiotics eradicate the bacteria, which can lead to an increased release of toxins that exacerbate the course of the disease.
- Monitor for the possible development of HUS.
- In the case of HUS, monitor and correct:
- Fluid status abnormalities
- Electrolyte disturbances
- Acid-base abnormalities
- Blood pressure
- RBC transfusions
- Up to 50% of HUS patients require dialysis.
- Avoid antiperistaltic agents (e.g., diphenoxylate/atropine) since they increase the risk of systemic complications.
References
- Murray, P. R., Rosenthal, K. S., & Pfaller, M. A. (2013). Medical microbiology. Philadelphia: Elsevier/Saunders
- Parija S.C. (2012). Textbook of Microbiology & Immunology.(2 ed.). India: Elsevier India.
- Sastry A.S. & Bhat S.K. (2016). Essentials of Medical Microbiology. New Delhi : Jaypee Brothers Medical Publishers.
- https://cmr.asm.org/content/11/1/142
- https://www.amboss.com/us/knowledge/Diarrheagenic_E._coli
- https://www.amboss.com/us/knowledge/Hemolytic_uremic_syndrome#xid=rL0f_g&anker=Z759bb631d23a3535c2b55dfd91c4807b
- https://healthywa.wa.gov.au/Articles/S_T/Shiga-toxin-producing-E-coli-STEC-and-haemolytic-uraemic-syndrome-HUS
