Serratia marcescens is a Gram-negative, motile, non-endospore-forming, rod-shaped, Gammaproteobacteria in the family Yersiniaceae in the Enterobacterales order.
S. marcescens was first identified as the culprit of an incidence of polenta discoloration (development of blood-red color in polenta (a boiled cornmeal dish)) in the city of Padua, Italy in 1819 by the Venetian pharmacist Bartolomeo Bizio. In 1823, he named the bacteria ‘Serratia marcescens’ in honor of his late friend Serafino Serrati. Although the name was then altered to Monas prodigiosus and Bacillus prodigiosus, Serratia marcescens was once more given to the species in the 1920s.
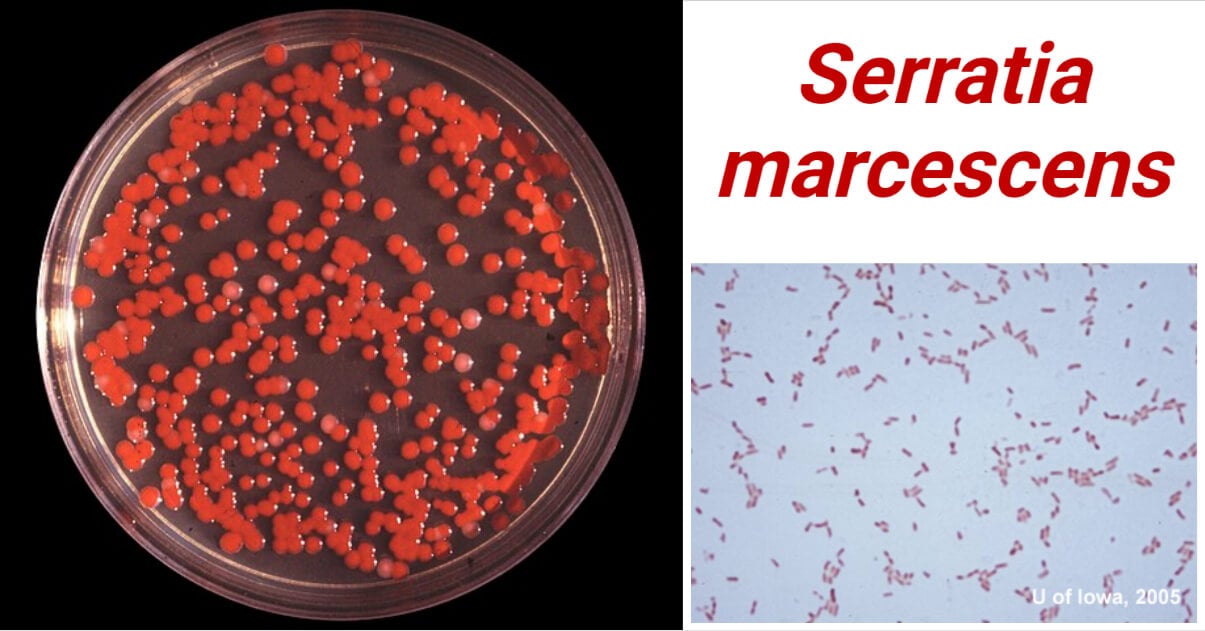
It is now regarded as one member of the coliform group. S. marcescens has been found to be an opportunistic pathogen, particularly in hospital settings where it can cause a variety of illnesses, including urinary tract infections, wound infections, and bloodstream infections, despite occasionally being a benign commensal bacterium. It is a major problem in healthcare settings due to its capacity to create a variety of virulence factors and its resistance to numerous antibiotics.
Interesting Science Videos
Classification of Serratia marcescens
The position of Serratia marcescens within bacterial taxonomy is a topic of some discussion. In the past, it was categorized as belonging to the family Enterobacteriaceae (even in Bergey’s manual of systematic bacteriology), although other investigations have suggested that it belongs in the family Yersiniaceae instead. In their 2006 book “The Genus Serratia,” Grimont and Grimont offered one of the initial suggestions to shift Serratia from the Enterobacteriaceae family to the Yersiniaceae family. Later, in a 2013 study in the Journal of Clinical Microbiology, Serratia marcescens was more connected to Yersinia species than to other Enterobacteriaceae family members. Although there is still debate in the classification, we are classifying Serratia in the Yersiniaceae family in this note.
| Domain | Bacteria |
| Phylum | Pseudomonadota |
| Class | Gammaproteobacteria |
| Order | Enterobacterales |
| Family | Yersiniaceae (Previously Enterobacteriaceae) |
| Genus | Serratia |
| Species | S. marcescens |
Habitat of Serratia marcescens
- S. marcescens is mesophilic saprophytic bacteria widely found in the environment. It is mainly found in moist damp places like bathrooms, basins, and water outlet areas in kitchens and other swampy areas with decaying vegetation. It is also found in soil and in association with insects, plants, and animals including humans.
- In humans, mainly in hospitalized ones, they are found in the upper respiratory tract, urinary tract, as well as in gastrointestinal tract.
- It is frequently found in healthcare settings. S. marcescens is frequently discovered in healthcare environments on surfaces like hospital equipment, sinks, and floors as well as on the skin and feces of healthcare personnel. It is known to leave behind biofilms on surfaces, which can make it challenging to remove using conventional cleaning techniques.
Morphology of Serratia marcescens
- They are rod-shaped (bacilli), non-motile non-endospore-forming bacteria. They measure about 1 to 2 μm in length and about 0.5 to 0.8 μm in diameter. (However, the size may vary according to growth conditions.)
- Under the microscope, they are seen as short straight, or slightly curved rods; often arranged in short chains.
- They often are capsulated. This capsule boosts their biofilm-forming ability as well as increases virulence.
- One of the distinguishing characteristics of S. marcescens is the production of the prodigiosin pigment at room temperature giving the characteristic red pigmentation. The pigment makes the surface where the bacteria are grown red in color.
Cultural Characteristics of Serratia marcescens
- Serratia marcescens is a non-fastidious bacterial species growing on a wide variety of substrates. It is naturally found in an area with an abundant organic substrate like decaying vegetation, and food, and in association with biotics as well as in harsh nutrition-deficient places like soil, bathroom tiles, distilled water, and some disinfectants. They are mesophilic bacteria and can grow from a temperature range of 5°C to 40°C; however, their ambient growth is seen at 37±2°C.
- Serratia marcescens is well known for the production of bright red pigment, prodigiosin. Although, this pigment is produced only at room temperature, 20 to 30°C. Besides, there are no other specific cultural characters.
- In the laboratory, Serratia marcescens is isolated and grown on a wide variety of general-purpose media like Nutrient Agar (NA), Tryptic Soya Agar (TSA), MacConkey Agar (MCA), Eosin Methylene Blue (EMB) Agar, Blood Agar (BA), Cetrimide Agar (CA), etc. There are some selective and chromogenic media as well as Brilliance Serratia Agar, ChromID Serratia Agar, caprylate-thallous (CT) mineral salts CT agar, etc.
Cultural characters of Serratia marcescens in some of these media are listed below:
- Nutrient Agar (NA):
On the nutrient agar, S. marcescens produce smooth, circular, and raised non-lactose fermenting colonies of around 2 to 4 mm. When grown at 20-30°C, red pigmentation can be observed after overnight (24 hours) incubation.
- Tryptic Soya Agar (TSA):
On the tryptic soya agar, S. marcescens produce smooth, circular, moist, and raised non-lactose fermenting colonies of around 2 to 4 mm. Production is prodigiosin giving bright red color can be observed on incubation at 20-30°C.
- MacConkey Agar (MAC):
On MacConkey Agar, S. marcescens produce smooth, raised, circular (2 to 4 mm), and non-lactose fermenting pink to red colonies.
- Blood Agar (BA):
On MacConkey Agar, S. marcescens produce Beta-hemolytic with a narrow zone of hemolysis, medium-sized (2 to 4 mm) circular grayish colonies. Red-color production is not clearly visible in BA, but, the bacterium produces prodigiosin even in BA if incubated at 30°C or below.
- Caprylate-thallous Agar Medium (CT Agar):
It is the highly selective and differential medium used to isolate Serratia spp. and differentiate them into different species. Serratia produces small slightly bluish-white colonies. S. marcescens produce red pigment at a suitable temperature.
- CHROMagar™ Serratia
It is a chromogenic medium used as a selective and differential chromogenic media for isolation and primary identification of S. marcescens. S. marcescens produces about 2 mm, circular Green-blue to metallic blue colonies.
Biochemical Characteristics of Serratia marcescens
General Biochemical Tests Results
| S.N. | General Biochemical Characteristics | Serratia marcescens |
| 1 | Capsule | Mostly Negative (Few are capsulated) |
| 2 | Catalase | Positive (+) |
| 3 | Citrate (Simmons) | Positive (+) |
| 4 | Coagulase | Negative (-) |
| 5 | Deoxyribonuclease (DNase) | Positive (+) |
| 6 | Flagella | Positive (+) |
| 7 | Gas | Variable (±) |
| 8 | Gram Staining | Gram Negative Bacilli |
| 9 | H2S (Hydrogen Sulfide) | Negative (-) |
| 10 | Indole | Negative (-) |
| 11 | Motility | Motile |
| 12 | Methyl Red (MR) | Negative (-) |
| 13 | Nitrate Reduction | Positive (+) |
| 14 | Oxidase | Negative (-) |
| 15 | OF (Oxidative Fermentation) | Facultative Anaerobes |
| 16 | Pigmentation | Positive (+) |
| 17 | Spore | Negative (-) |
| 18 | Triple Sugar Iron (TSI) | K/A (Alkaline/Acidic) (Red/Yellow) |
| 19 | Urease | Negative (-) |
| 20 | Voges Proskauer (VP) | Positive (+) |
Carbohydrate Fermentation Tests
| S.N. | General Biochemical Characteristics | Serratia marcescens |
| 1 | Adonitol | Positive (+) |
| 2 | Arabinose | Negative (-) |
| 3 | Arabitol | Variable |
| 4 | Cellobiose | Negative (-) |
| 5 | Dulcitol | Negative (-) |
| 6 | Fructose | Positive (+) |
| 7 | Galactose | Positive (+) |
| 8 | Glucose | Positive (+) |
| 9 | Glycerol | Positive (+) |
| 10 | Lactose | Negative (-) |
| 11 | Maltose | Positive (+) |
| 12 | Mannitol | Positive (+) |
| 13 | Mannose | Positive (+) |
| 14 | Rhamnose | Negative (-) |
| 15 | Ribose | Positive (+) |
| 16 | Sorbitol | Positive (+) |
| 17 | Sucrose | Positive (+) |
| 18 | Trehalose | Positive (+) |
| 19 | Tartrate | Negative (-) |
| 20 | Xylose | Negative (-) |
Enzymatic Hydrolysis Tests
| S.N. | General Biochemical Characteristics | Serratia marcescens |
| 1 | Acetate Utilization | Variable |
| 2 | Arginine Dehydrolase | Negative (-) |
| 3 | Casein Hydrolase | Positive (+) |
| 4 | DNase | Positive (+) |
| 5 | Esculin Hydrolysis | Positive (+) |
| 6 | Gelatinase | Positive (+) |
| 7 | Lipase | Positive (+) |
| 8 | Lysine Decarboxylase | Positive (+) |
| 9 | ONPG (β-Galactosidase) | Positive (+) |
| 10 | Ornithine Decarboxylase | Positive (+) |
| 11 | Phenylalanine Deaminase | Negative (-) |
| 12 | Tryptophanase | Negative (-) |
Virulence Factors of Serratia marcescens
- Extracellular Enzymes:
Serratia marcescens produces a number of extracellular enzymes, such as lipases, proteases, and DNases, that can damage host tissues and enable bacterial invasion.
- Hemolysin:
Hemolysin, a pore-forming toxin made by Serratia marcescens, can lyse host cells including red blood cells. Hemolysin can harm host tissues and aid in the development of abscesses.
- Lipopolysaccharide (LPS):
Some Serratia marcescens strains have a changed LPS structure that helps the bacteria become more virulent and avoid being detected by the human immune system. One kind of LPS produced by Serratia marcescens is capable of triggering host immunological reactions and causing inflammation.
- Siderophores:
Serratia marcescens produce siderophores, which help the bacterium to scavenge iron from host tissues and promote growth and survival. Serratiochelin, a form of siderophore produced by some Serratia marcescens strains, can also increase bacterial pathogenicity.
- Flagella:
Because Serratia marcescens has flagella, it can travel across the host’s tissues and evade immune responses. Additionally, flagella can facilitate bacterial adherence to host cells and aid in the development of biofilms.
Pathogenesis of Serratia marcescens
Several virulence factors, including adhesins, biofilm formation, motility, and the generation of exoenzymes and exotoxins, play a role in S. marcescens‘ pathogenicity. S. marcescens is able to colonize, persist, and cause illness and tissue damage because of these virulence factors.
Pathogenesis varies depending on the site of infection. However, general pathogenesis can be summarized in the following steps:
Adhesion and Colonization
S. marcescens attach to catheters or other medical devices inside the human body or attach to the cells and tissues of the host. Adhesins help in their attachment. Once attached, they multiply rapidly and colonize the attached surfaces.
Biofilm Formation
Serratia marcescens can form biofilm on the surface of several medical devices and catheters. Biofilm formation will allow them to attach more firmly to the host’s surface, adjust to changing environments, as well as act as a barrier against some host’s immune components.
Infection Development
Once S. marcescens colonize and multiply inside host tissue, they begin to develop clinical manifestations. Production of exoenzymes and exotoxins helps to damage the host’s tissues and develop an infection. The pathogenesis varies depending on the site of infection.
Clinical Manifestation of Serratia marcescens
S. marcescens demonstrate a wide range of clinical manifestations in humans. They are typically reported in healthcare-associated infections; however, community-acquired infections are also common. S. marcescens are opportunistic pathogens meaning they mainly infect persons with compromised or weak immune systems. Some common diseases caused by S. marcescens in humans are listed below:
- Urinary Tract Infections (UTIs):
S. marcescens is a common causative agent of UTIs, especially healthcare-associated UTIs in patients with indwelling urinary catheters.
- Respiratory Tract Infections (RTIs):
In patients with compromised immune systems and/or underlying respiratory diseases like COPD, S. marcescens is reported to cause pneumonia. Rarely, community-acquired pneumonia is also reported.
- Wound Infection:
Several cases of hospital-acquired soft tissue infections, particularly surgical site infections in patients with gastrointestinal and urinogenital surgeries, are associated with S. marcescens.
- Bloodstream Infections (BSIs):
S. marcescens can cause BSIs in hospitalized patients with catheters, patients undergone invasive medical treatments, and immunocompromised patients.
- People with S. marcescens-associated BSIs may also experience other conditions like endocarditis, osteomyelitis, meningitis, etc. Meningitis is mainly seen in neonates and immunocompromised patients.
Laboratory Diagnosis of Serratia marcescens
Cultural Characteristics and Morphological Identification
- In the lab, MacConkey Agar and Blood Agar are commonly used for the primary isolation of S. marcescens from clinical specimens. Colony characteristics and production of red pigment when incubated at 300C are studied for primary identification of S. marcescens.
- In MAC agar medium, they produce medium-sized, circular, smooth, non-lactose fermenting colonies. In blood agar, a narrow zone of hemolysis is observed around medium-sized (2 to 4 mm) greyish-white colonies. They produce red pigment when incubated at around 300C.
- Under the microscope, they appear as Gram-negative rod-shaped bacteria, typically arranged in chains.
Biochemical Identification
- The isolated colonies are subjected to biochemical tests like the IMViC test, Oxidase test, Catalase test, urease test, TSI test, SIM test, and other carbohydrate fermentation tests.
- API 20E Rapid System:
Based on its biochemical profile, Serratia marcescens can be quickly identified using the API 20E system, a commercial test kit.
Molecular Identification
Serratia marcescens can be positively identified in the lab using PCR-based techniques such as 16S rRNA gene sequencing and multilocus sequence typing.
Treatment Options for Serratia marcescens Infections
Antibiotics such as carbapenems, aminoglycosides, fluoroquinolones, and third-generation cephalosporins are frequently used to treat Serratia marcescens infections. However, Serratia marcescens isolates from various sources across the world have been found to be resistant to these drugs.
Antibiotic Resistance Profile of Serratia marcescens
- It is naturally resistant to a number of antibiotics. Serratia marcescens is known to gain resistance to additional antibiotics through a variety of methods, including mutation, horizontal gene transfer, and overexpression of efflux pumps. As a result, Serratia marcescens infections can be difficult to treat, and antibiotic selection should be based on susceptibility testing.
- Serratia marcescens is also known to produce various beta-lactamases. Extended-spectrum beta-lactamases (ESBLs), which may hydrolyze beta-lactam antibiotics like penicillins and cephalosporins and cause resistance, are among the beta-lactamases that Serratia marcescens. Additionally, Serratia marcescens is capable of producing carbapenemases that confer resistance to carbapenems, such as KPC (Klebsiella pneumoniae carbapenemase) and IMP (imipenemase).
- Serratia marcescens strains that are multi- and pan-drug resistant (MDR and PDR, respectively) have arisen recently, further limiting available treatments.
Prevention of Serratia marcescens infections
S. marcescens is an opportunistic pathogen and is mainly involved in healthcare-associated infections. Infection from S. marcescens can be prevented by following practices like good hand hygiene, environmental cleaning, contact precaution, sterilization, use of personal protective equipment, surveillance, provision of education and training to healthcare workers, etc.
References
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/serratia
- Forbes, B. A., Sahm, D. F., & Weissfeld, A. S. (Eds.). (2007). Bailey & Scott’s Diagnostic Microbiology (12th ed.). Mosby Elsevier.
- Versalovic, J., Carroll, K. C., Funke, G., Jorgensen, J. H., Landry, M. L., & Warnock, D. W. (Eds.). (2011). Manual of Clinical Microbiology (10th ed.). ASM Press.
- Shimuta, K., Ohnishi, M., Iyoda, S. et al. The hemolytic and cytolytic activities of Serratia marcescensphospholipase A (PhlA) depend on lysophospholipid production by PhlA. BMC Microbiol 9, 261 (2009). https://doi.org/10.1186/1471-2180-9-261
- https://www.chromagar.com/en/product/chromagar-serratia/
- Pérez-Viso, B., Aracil-Gisbert, S., Coque, T. M., Ruiz-Garbajosa, P., & Cantón, R. (2021). Evaluation of CHROMagar™-Serratia agar, a new chromogenic medium for the detection and isolation of Serratia marcescens. European Journal of Clinical Microbiology & Infectious Diseases, 40(12), 2593-2596. https://doi.org/10.1007/s10096-021-04328-w
- Starr MP, Grimont PA, Grimont F, Starr PB. Caprylate-thallous agar medium for selectively isolating Serratia and its utility in the clinical laboratory. J Clin Microbiol. 1976 Sep;4(3):270-6. doi: 10.1128/jcm.4.3.270-276.1976. PMID: 972193; PMCID: PMC274449.
- Mahlen, S. D. (2011). Serratia Infections: From Military Experiments to Current Practice. Clinical Microbiology Reviews, 24(4), 755-791. https://doi.org/10.1128/CMR.00017-11
- Holt, J. G., Krieg, N. R., Sneath, P. H. A., Staley, J. T., & Williams, S. T. (1994). Bergey’s manual of determinative bacteriology (9th ed.). Williams & Wilkins
- Hori, K., Miyata, R., Kozuka, S., Misawa, N., & Uemura, T. (2018). Hemolysin production by Serratia marcescens is controlled by the quorum sensing system and the RssAB two-component system. Microbiology and immunology, 62(7), 464-472.
- Parsek, M. R., & Singh, P. K. (2003). Bacterial biofilms: an emerging link to disease pathogenesis. Annual review of microbiology, 57, 677-701.
- https://microbiologyinfo.com/biochemical-test-and-identification-of-serratia-marcescens
