Carbohydrates are the major source of energy in the body, according to the American Diabetes Association. Since they have carbon, hydrogen, and oxygen as chemical constituents, roughly (C.H₂O)n where n≥3, they are known as carbohydrates. Sugars, fibers, and starches, found in fruits, vegetables, grains, and milk products, are examples of carbohydrates.
- Monosaccharides are the building blocks of carbohydrates. Many of these substances are formed through a process known as glyconeogenesis from simpler molecules. Others are byproducts of photosynthesis. These are classified according to the number of carbon atoms, such as triose for 3 C-atoms in the carbohydrate, tetrose for 4 C-atoms, pentose for 5 C-atoms, and so on. Some examples include glucose, fructose, galactose, etc.
- A small number of covalently bonded monosaccharide molecules make up an oligosaccharide. They frequently cooperate with proteins (glycoproteins) and lipids (glycolipids), serving both regulatory and structural roles. They contain 3-10 monosaccharide units linked together through glycosidic linkage. Examples are:
- Maltose (Glucose+Glucose)
- Sucrose (Glucose+ Fructose)
- Lactose (Glucose+Galactose)
- Raffinose (Glucose+Fructose+Galactose)
- Polysaccharides have molecular weights far into the millions of daltons and are made up of several covalently bonded monosaccharide units. These are also known as glycans which are further classified as homopolysaccharides or heteropolysaccharides whether they consist of one type or more than one type of monosaccharide residue.
- Glucans (Polymer of glucose)
- Galactans (Polymer of galactose)
- Heparin (Polymer of D-glucuronic acid, L-iduronic acid, N-sulfo-D-glucosamine)
- Hyaluronic acid (Polymer of D-glucuronic acid and N-acetyl-glycosamine)
Interesting Science Videos
What are Reducing Sugars?
Reducing sugars are carbohydrates that act as a reducing agent with a free aldehydic (-CHO) or ketonic (-CO-) group in its structure and get oxidized by weak oxidizing agents like salts of metals.
The presence of free carbon at the end of these reducing sugars is known as reducing ends. All categories of carbohydrates: monosaccharides, disaccharides, oligosaccharides, and polysaccharides include reducing sugars, whereas all monosaccharides, some disaccharides, some oligosaccharides, and some polysaccharides are reducing sugars.
- Two forms of sugars found in the monosaccharides, aldose, and ketose, are reducing sugars, as ketone groups are present in ketoses while aldehyde groups are present in aldoses. Examples of ketoses are fructose, and aldoses are glucose and galactose.
- Tautomerization is the process by which a compound’s isomers are changed into tautomers. Ketoses undergo tautomerization to form aldoses and then act as reducing sugars.
- Disaccharides can either be reducing or non-reducing. For example, Sucrose and trehalose are non-reducing sugars since glycosidic bonds between their anomeric carbons do not permit them to transform into an open-chain form with an aldehyde group. Instead, they remain in the cyclic form. However, lactose and maltose are reducing disaccharides.
Characteristics of Reducing Sugars
They can transfer electrons to other compounds and can cause the reduction of other compounds, themselves being oxidized. They serve as a reducing agent. The following points summarize some of the most essential qualities of reducing sugar.
- A free aldehyde group and a ketone, respectively, are present in the structures of reducing sugars such as glucose and fructose.
- Reducing sugar can be oxidized with weak oxidizing substances, such as metal salts, which can be used to oxidize them.
- When carbon is joined to a few oxygen molecules to generate alcohol or ether, a hemiacetal structure develops. Any sugar that contains a hemiacetal is a reducing sugar.
- Osazones are generated when mutarotation is produced in reducing sugars.
- The reducing agents in an aqueous solution often produce one or more compounds with an aldehyde group.
- Maillard’s reaction causes the browning of food items such as cakes, slices of bread, chocolates, coffee, and processed and baked meals due to the interaction between reducing sugars and amines. This reaction is evident when the food is heated for extended periods or remains at room temperature.
- Reducing sugars reduces cupric ions of Fehling’s solution and Tollen’s reagent into cuprous ions to form brick-red precipitates.
Examples of Reducing Sugars
All monosaccharides are reducing sugars. These contain free aldehyde (aldoses) and ketone (ketoses) groups. Ketone tautomerizes in solution to produce an aldehyde. Examples are glucose, galactose, fructose, ribose, glyceraldehyde, xylose, etc. The Aldehydic group readily undergoes oxidation to form carboxylic acid and reduce the other reactive agent in the process simultaneously.
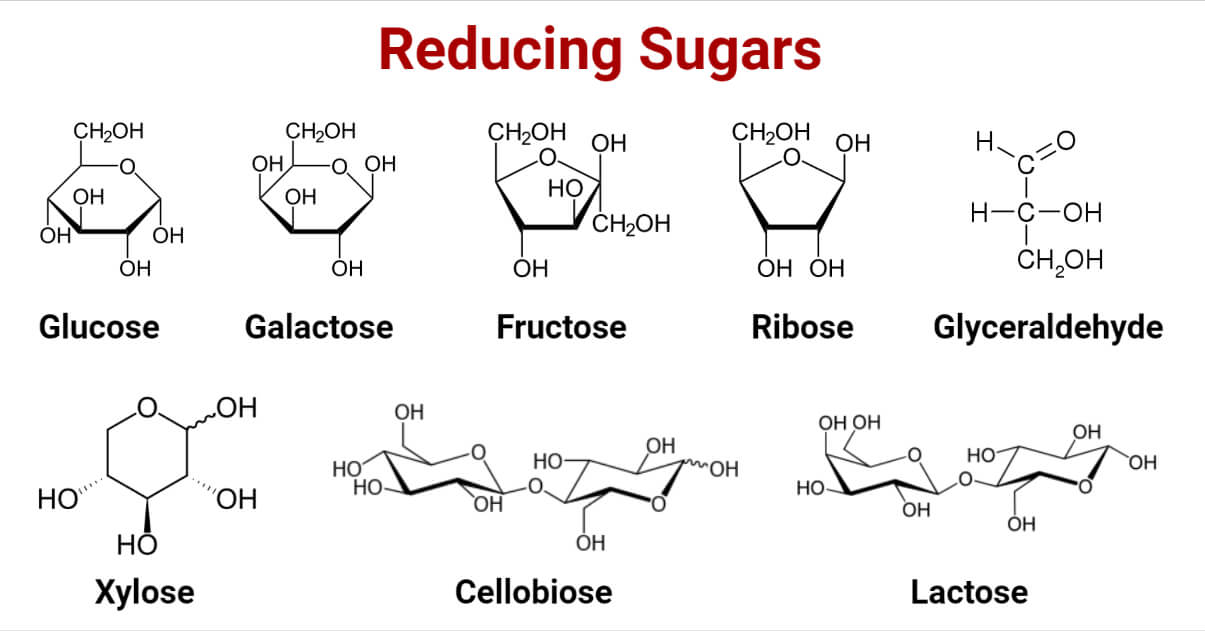
- Reducing disaccharides have a free hemiacetal unit as an aldehydic group in one of its monosaccharides. For example, Lactose, cellobiose, and maltose are reducing disaccharides whose one hemiacetal unit is free. At the same time, the other is occupied by the glycosidic bond, and the free hemiacetal unit facilitates it to act as a reducing agent.
- The nonreducing disaccharides and polysaccharides are acetals. Usually, complex polysaccharides have a single hemiacetal unit which is not enough for such a huge molecule to give a positive test for reducing sugars. That molecule which does not have any hemiacetal groups is reducing in nature.
Identifying Tests for Reducing Sugar
Commonly performed tests to identify whether there is the presence of reduced sugar or not in the sample are Benedict’s test and Fehling’s test.
Benedict’s Test
The test procedure begins with dissolving the food samples in water to determine whether reduced sugar is present. A very limited amount of Benedict’s reagent is added, and at this point, the solution starts to cool, followed by the onset of cooling of the solution. The solution begins to change its color after about 10 minutes. The presence of reducing sugar is indicated if the hue turns blue. However, if the hue shifts progress to green, yellow, orange, red, and finally to dark red or brown, that indicates that the food contains reducing sugar.
[ Benedict’s solution is the aqueous solution of anhydrous sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate.]
Fehling Test
The sample in which the presence of reducing sugar is to be detected is uniformly mixed in water, and then the warm Fehling’s solution is added. The presence of reducing sugar is confirmed by the change of the color of the solution into a red-brown rusty color.
[Fehling’s solution is prepared from an aqueous solution of potassium sodium tartrate tetrahydrate and copper II sulfate pentahydrate combined in equal parts.]
Applications of Reducing Sugars
Some of the utilization of reducing sugars are listed below:
- Reducing sugar consumption specifically lowers the chance of being overweight and obese, lowering the risk of getting diabetes.
- Additionally, it significantly lowers dental cavities.
- The quality of beverages is indicated by the level of reducing sugars.
References
- https://chem.libretexts.org/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Reducing_Sugar
- https://www.biologyonline.com/dictionary/reducing-sugar
- https://study.com/academy/lesson/reducing-vs-non-reducing-sugars-definition-comparison.html
- https://www.chem.ucalgary.ca/courses/351/Carey5th/Ch25/ch25-2-5.html
- https://doi.org/10.1007/978-1-4615-8146-8_6
- https://sciencing.com/sucrose-nonreducing-sugar-5882980.html
- https://www.masterorganicchemistry.com/2017/09/12/reducing-sugars/
- https://www.livestrong.com/article/386795-the-definition-of-reducing-sugars/
- https://researchtweet.com/reducing-sugar-definition-characteristic-examples/
- https://www.biologyexams4u.com/2012/10/differences-between-reducing-and-non.html?m=1
- Zoecklein, B.W., Fugelsang, K.C., Gump, B.H., Nury, F.S. (1990). Carbohydrates: Reducing Sugars. In: Production Wine Analysis. Springer, Boston, MA.

Greetings, thank you for providing this article. I believe you have the definition of reducing sugar slightly off and correcting would prevent confusion of the reader. The sentence I find problematic is “Reducing sugars are carbohydrates that can oxidize other substances by contributing electrons while being reduced.” A reducing sugar can reduce other substances via contributing an electron. This results in oxidation of the sugar, not reduction. Also correct the section “Characteristics of Reducing Sugars”.
Hi Andrew,
Thank you so much for the correction. I have re-written as follows:
Reducing sugars are carbohydrates that act as a reducing agent with a free aldehydic (-CHO) or ketonic (-CO-) group in its structure and get oxidized by weak oxidizing agents like salts of metals. They can transfer electrons to other compounds and can cause the reduction of other compounds, themselves being oxidized.