Interesting Science Videos
Fehling’s Test Definition
Fehling’s test is a chemical test used to differentiate between reducing and non-reducing sugars. This test can also be used to distinguish ketone functional group carbohydrates and water-soluble carbohydrates.
Objectives of Fehling’s Test
- To detect the presence of carbohydrates in a solution.
- To differentiate between reducing and non-reducing sugars.
Principle of Fehling’s Test
- The carbohydrates having free or potentially free carbonyl groups (aldehyde or ketone) can act as reducing sugars.
- The Fehling’s solution appears deep blue in color and consists of copper sulfate mixed with potassium sodium tartrate and strong alkali, which is usually sodium hydroxide.
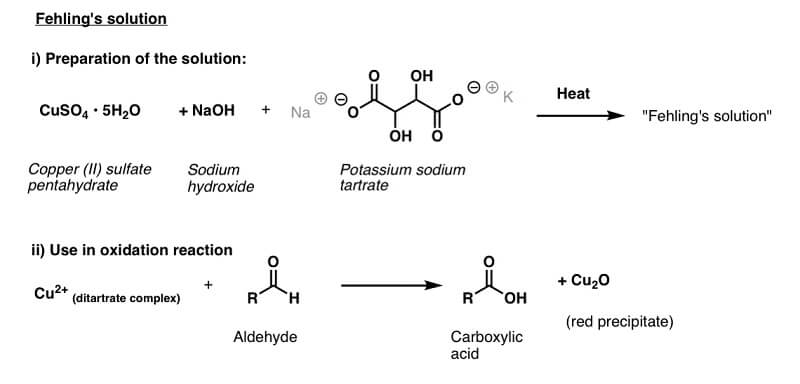
Image Source: Master Organic Chemistry.
- On heating, the sample with the Fehling’s solution, bistartarocuprate (II) complex oxidizes the aldoses to corresponding aldonic acids.
- In the process, the copper (II) ions of the complex are reduced to insoluble yellow or red-colored precipitate or cuprous (I) oxide (Cu2O) ions.
- The ketones, on the other hand, are oxidized to yield shorter chains of acids.
- The tartrate ions prevent the formation of insoluble Cu(OH)2 from the reaction of copper sulfate and sodium hydroxide present in the solution by forming a bistartarocuprate (II) complex.
- This complex releases cupric ions slowly for reduction, thus preventing the formation of black cupric oxide.
- If Fehling’s solution is heated in the absence of reducing sugars, it forms a black precipitate of cupric oxide.
Reaction
2Cu(OH)2 + reducing sugar → 2Cu2O + Aldonic acid
Requirements
1. Reagent
- Fehling’s solution A: Dissolve 7 g of CuSO4.7H2O in 100 ml of water.
- Fehlings solution B: Dissolve 24 g of KOH and 34.6 g of potassium sodium tartrate in 100 ml water.
- Fehling’s solution: Mix equal volumes of both the solution just before use.
- Sample (5% Glucose, 5% Sucrose, 5% Fructose, 5% Starch, 5% lactose)
2. Materials Required
- Pipettes
- Test tubes
- Test tube stand
3. Equipment
- Water bath
Procedure of Fehling’s Test
- Take 1 ml of a given sample in a clean, dry test tube. The concentration of the test samples should be 5% (w/v).
- Take control of 1 ml of distilled water in another tube.
- Add about 2-3 drops of Fehling’s reagent to both the tubes and mix them in a vortex.
- Keep the test tubes in the water bath for 1-2 minutes.
- Observe the appearance of color in the test tubes.
- Note down the appearance of color seen in the test tubes.
Result and Interpretation of Fehling’s Test
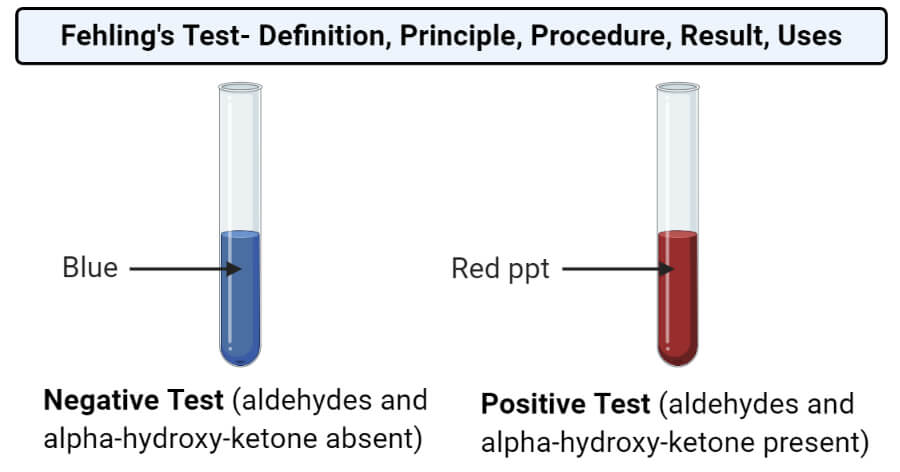
- The appearance of a reddish-brown precipitate indicates a positive result and the presence of reducing sugars.
- The absence of the reddish precipitate or the appearance of deep blue color indicates a negative result and lack of reducing sugars.
Uses of Fehling’s Test
- Fehling’s test is used to distinguish between the presence of aldehydes and ketones in carbohydrates as ketone sugars except alpha-hydroxy-ketone do not react in this test.
- Fehling’s test is performed in medical facilities to detect the presence of glucose in urine. This helps to identify whether the patient has diabetes or not.
Limitations of Fehling’s Test
- Aromatic aldehydes cannot be detected by this test.
- This reaction takes place only in an alkaline environment. In an acidic environment, the copper (II) ions would be stabilized and not easily oxidized, thus failing the reaction.
References and Sources
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- 3% – https://www.researchgate.net/publication/313745155_Practical_Biochemistry_A_Student_Companion
- 3% – https://www.answers.com/Q/What_is_the_role_of_sodium_potassium_tartrate_in_Fehling%27s_solution
- 2% – https://en.wikipedia.org/wiki/Fehling%27s_solution
- 2% – https://byjus.com/jee/fehlings-solution/
- 2% – https://byjus.com/chemistry/fehling-test/
- 2% – http://www.bioline.org.br/request?tc09039
- 1% – https://www.medicalnewstoday.com/articles/323378
- 1% – https://encyclopedia2.thefreedictionary.com/carbohydrate
- 1% – https://chemdemos.uoregon.edu/demos/Fehling-Test
- 1% – https://byjus.com/chemistry/tests-for-aldehydes-and-ketones/
- 1% – http://wwwchem.uwimona.edu.jm/courses/Fehling.html

Great piece of work. Looking forward to more articles
Too beautiful dear keep it up
Good and precise awesome work….👍🥳🥳