Interesting Science Videos
What is Benedict’s Test?
Definition of Benedict’s Test
Benedict’s Test is a chemical analytical method used for the detection of reducing sugar in a solution. Benedict’s Test is a qualitative test often used for the differentiation of carbohydrates (saccharides/sugars) into reducing and non-reducing types.
Reducing sugars are those sugars that have free aldose or ketose groups capable of donating electrons to other molecules oxidizing them. They have free carbon at the end of their molecules. All monosaccharides and some disaccharides, oligosaccharides, and polysaccharides are reducing sugar.
It is widely used to identify monosaccharides (simple sugars) and other reducing sugars. It is used as an alternative to Fehling’s test. Identification is based on the development of brick-red color due to the chemical reaction between Benedict’s reagent and reducing sugar. Based on the intensity of the reaction mixture, the concentration of sugar can be determined, but numerical value can’t be estimated. Hence, it is a qualitative and semi-quantitative test.
It is also used for detecting glucose in urine as a presumptive test of diabetes mellitus.
It was discovered by American Chemist/Biochemist Stanley Rossiter Benedict.
Objectives of Benedict’s Test
- To detect the presence of reducing sugar in the sample solution
- To diagnose diabetes mellitus by detecting glucose in the urine sample
- To estimate the concentration of reducing sugar in the sample solution
- To differentiate and identify the extracted carbohydrates
Principle of Benedict’s Test
Sodium carbonate in the Benedict reagent increases the pH of the sample-reagent solution mixture. Under warm alkaline conditions reducing sugars are tautomerism to strong reducing agents, enediols. These enediols reduce the cupric ions (Cu2+) (present as Copper Sulfate (CuSO4)) of Benedict reagent into cuprous ions (Cu+). The cuprous particles are present in form of insoluble Copper (I) oxide or cuprous oxide (Cu2O) which is of red color. These red-colored copper oxides get precipitated.
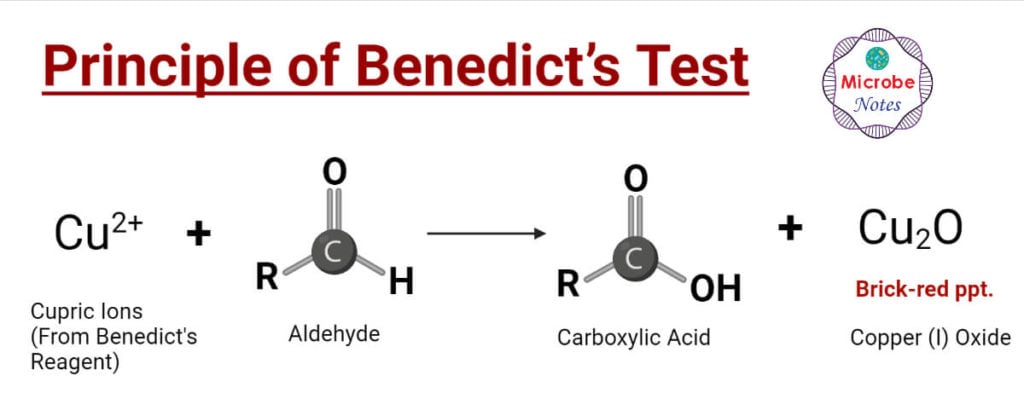
The concentration of reducing sugar in the sample differs from the intensity and shade of the color of the reaction mixture. This shade of color can be used to estimate the concentration of reducing sugar in the sample. Color may vary from greenish to yellow to orange-red to brick-red. As the concentration of reducing sugar increases color gradually changes from greenish to yellowish to orange to brick-red.
Requirements of Benedict’s Test
- Sample solution of unknown carbohydrate (or urine sample)
- Test-tubes and test-tube holders
- Pipette
- Bunsen burner
- Benedict’s Reagent
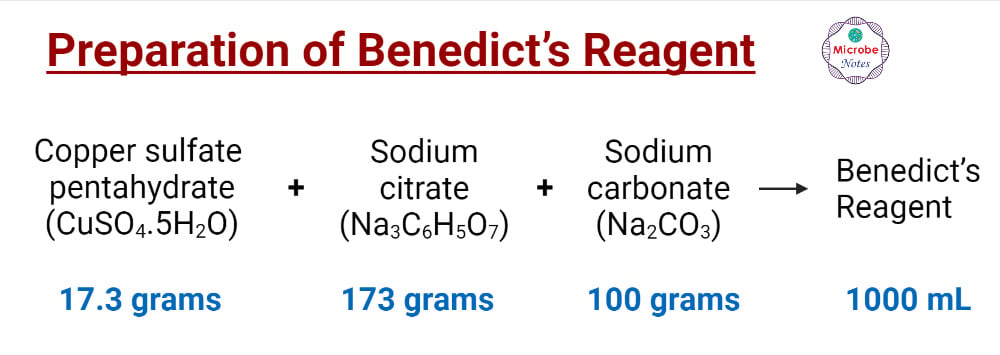
Preparation of Benedict’s Reagent
- Measure 17.3 grams of copper sulfate (CuSO4), 173 grams of sodium citrate (Na3C6H5O7), and 100 grams of anhydrous sodium carbonate (Na2CO3) (or 270 grams of sodium carbonate decahydrate (Na2CO3.10H2O))
- Put all the measured chemicals in a volumetric flask of 1000 mL.
- Pour distilled water up to 1000 mL marking.
- Dissolve all the components properly by shaking gently.
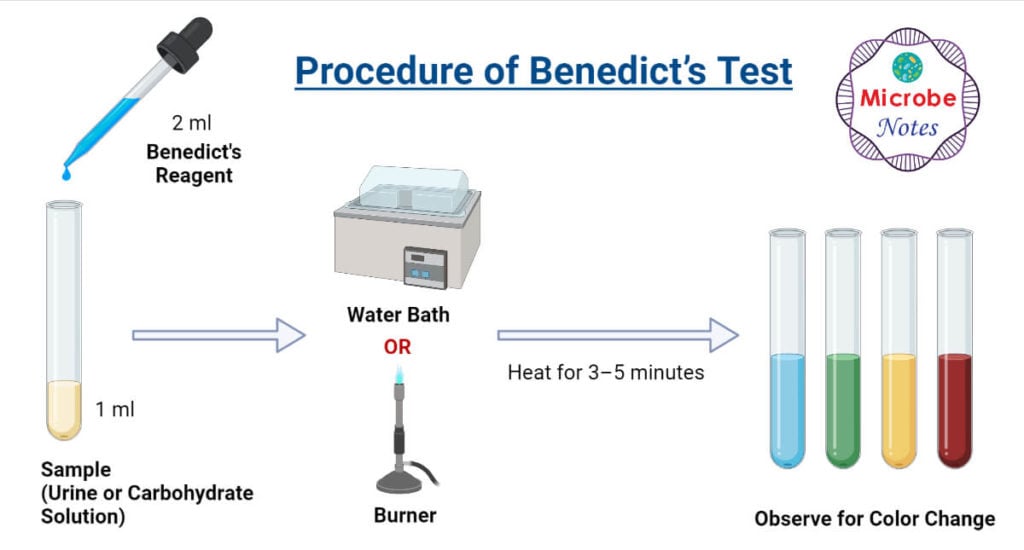
Procedure of Benedict’s Test
- In a clean test tube add 1 mL of sample solution (urine or carbohydrate solution).
- Add 2 mL of Benedict’s reagents over the sample.
- Place the test tube over a boiling water bath and heat for 3–5 minutes or directly heat over a flame.
- Observe for color change.
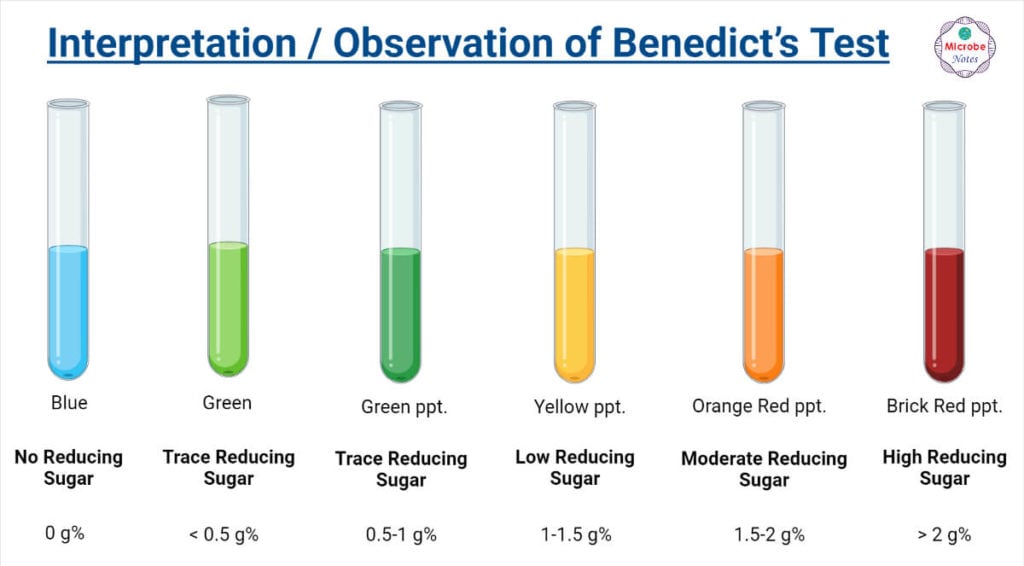
Result Interpretation / Observation of Benedict’s Test
Any change in color from blue to green or yellow or orange or red within 3 minutes indicates a positive Benedict test i.e. presence of reducing sugar in the sample.
For semiquantitative evaluation, the concentration of reducing sugar can be estimated based on the shade of developed color as follows;
| Shade of Color | Approx. Concentration of Reducing Sugar (in g%) | Indication |
| Blue | 0 | No reducing sugar |
| Green solution | < 0.5 | Trace reducing sugar |
| Green ppt. | 0.5 – 1 | Trace reducing sugar |
| Yellow ppt. | 1 -1.5 | Low reducing sugar |
| Orange-red ppt. | 1.5 – 2 | Moderate reducing sugar |
| Brick-red ppt. | >2 | High reducing sugar |
Precautions of Benedict’s Test
- Measurement must be accurate.
- Don’t heat the mixture quickly. It is best to heat over a water bath slowly.
- During heating the solution, use a test-tube holder.
- Don’t face the test tube towards oneself or others during heating.
- Heating should be done at least thrice before reporting negative.
Applications of Benedict’s Test
- In biochemistry for analysis and identification of unknown carbohydrate extracts.
- In clinical diagnosis for rapid presumptive diagnosis of diabetes mellitus.
- In quality control for detecting simple sugar and their quantification.
Advantages of Benedict’s Test
- A simple test requiring fewer materials and less time.
- Non-toxic reagents.
- Inexpensive.
- Both qualitative and semi-quantitative evaluation.
Limitations of Benedict’s Test
- False-positive result due to reaction with drugs like penicillin, isoniazid, streptomycin, salicylates, and p-aminosalicylic acid.
- Chemicals in urine like creatinine, ascorbic acid, and urate retard Benedict’s reaction.
- The exact concentration of reducing sugar can’t be measured; only an estimated semiquantitative value can be indicated.
- Requires further test for identification of the carbohydrate .
References
- Robert D. Simoni; Robert L. Hill & Martha Vaughan (2002). “Benedict’s Solution, a Reagent for Measuring Reducing Sugars: the Clinical Chemistry of Stanley R. Benedict”. J. Biol. Chem. 277 (16): 10–11. doi:10.1016/S0021-9258(19)61050-1.
- National Institutes of Health, Testing for Lipids, Proteins and Carbohydrates – Benedict’s solution.
- Northern Kentucky University- Benedict’s Reagent: A Test for Reducing Sugars.
- Shrestha B (2002). Practical biochemistry and biotechnology. First edition. 99933-665-1-X.
- Fayetteville State University- Biological Molecules: Carbohydrates, Lipids, Proteins.
- KNUST Open Educational Resources.
- Amrita Virtual Lab Collaborative Platform- Qualitative Analysis of Carbohydrates.
- Benedict’s Test – Reagent Preparation, Principle, Procedure, Reaction (byjus.com)
- Benedicts Test – Principle, Procedure, Result and Limitation (vedantu.com)
- Benedict’s Test- Principle, Preparation, Procedure and Result Interpretation (microbiologyinfo.com)
- Benedict’s test: Definition, Principle, Uses, and Reagent (chemistrylearner.com)

Why we use 1.5 ml of Benedict’s reagent for 1 ml of glucose
Teacher’s notes or Teacher’s suggestion should be included for more perfect.
Thank you for sharing. It is complete and simple explanation.
Please kindly share another practical paper for IGCSE A level biology.
The concentration of reducing sugar (g%)….what is the meaning of g%?
I am trying to find out what it means too.
Hi Russell,
g% means gram percentage. I mean the number of grams of solute per 100 ml or 100 grams of solution. 🙂
For Example- 2.5g% means there are 2.5 grams of reducing sugar in 100 ml of solution.
Thank you for responding this was a big help! 😀
Hi Sourav,
g% means gram percentage. I mean the number of grams of solute per 100 ml or 100 grams of solution. 🙂
For Example- 2g% means there are 2 grams of reducing sugar in 100 ml of solution.
hi im syahmi iqbal, thank you for the web site. it really helps me a lot for my assignment.
Thank you, Happy that our website is helping you in your assignment.