KOH test, also known as KOH mount or KOH preparation, is a rapid test used to visualize the fungal structures in clinical samples using potassium hydroxide (KOH) as a clearing reagent.
Microscopic observation of fungal structure is an important method to identify fungal species. In clinical laboratories, microscopic observations of clinical specimens are performed to reveal the presence of fungi together with their structure before culturing or subjecting the specimen to any testing methods of fungal identification. India ink preparation, Giemsa staining, periodic-acid-Schiff staining, Grocott’s methenamine silver stain staining, calcofluor mount, and KOH mount are routinely used in diagnostic labs for microscopic examination of clinical specimens.
Mycoses are rapidly diagnosed by observing the presence of fungal pathogens in a clinical sample. This will guide the microbiologist about fungal morphology and helps in making presumptive identification of fungal genera and also helps to determine the need for culture and types of culture media to be used. Being the easiest, simplest, and cheapest fungal observation technique, it is widely used in diagnostic laboratories.
Interesting Science Videos
Objectives of KOH Test
- To visualize fungi and their structure in a clinical specimen.
- To make presumptive identification of dermatophytes.
- To make a preliminary diagnosis of mycoses.
Principle of KOH Test
The potassium hydroxide dissolves proteinaceous substances including keratin, adhesives that hold keratinized cells together, and other alkali-sensitive tissue materials in clinical specimens. This digestion results in the breakdown of the cellular components; hence makes the specimen transparent, releases the bound fungal components, and makes the fungal elements clearly visible. The fungal components, however, are alkali resistant so they remain intact. These allow clear visualization of the microscopic morphology of the fungi and fungal elements in the sample.
Requirements
a. Reagents
10% to 30% KOH solution is used as a reagent for the KOH mount test.
Composition and Preparation of KOH Solutions (100 mL)
| Weight of KOH Pellets (in grams) | The volume of Distilled Water (in mL) | Final Concentration of KOH Solution (w/v) (in percentage) |
| 10 | 90 | 10% |
| 20 | 80 | 20% |
| 30 | 70 | 30% |
Preparation
- In a sterile beaker, add the appropriate amount of KOH pellet and pour the required amount of sterile distilled water to make the KOH solution of the required concentration. (Refer to the above table for the measure of KOH and water.)
- Shake the mixture properly to completely dissolve the KOH pellet. (Use a magnetic stirrer or shaker if available.)
b. Equipment
| Petri Plates Test tubes | Weighing Machine Microscope | Bunsen burner Glass slide and cover slips | Pipette |
PPE and other general laboratory materials
c. Sample/Specimens
All types of clinical specimens can be used for KOH mount. Generally, the specimens include skin scrapings, nail clips, hair, pus, sputum, CSF, tissue (biopsy) sample, urine, mucous membrane swabs, bronchial and alveolar washings, etc.
Specimens determine the concentration of KOH be used. For sputum, pus, CSF, urine, and swabs 10% KOH is effective enough to dissolve tissue debris. For skin scrapings, some swabs with lots of tissue debris, tissue samples, and thick pus, 20% KOH will do the job better and quicker. And for sturdy samples like nail clippings, and hair 30% KOH should be used to get a quick and complete dissolution of debris and keratins.
Procedure of KOH Test
a. KOH Mount Method
- In a clean and sterile glass slide, place a drop of KOH. (Concentration of KOH depends on the specimen.)
- Transfer a small portion of the specimen over the KOH drop and place it on the cover slip on top.
- Incubate the specimen-KOH mixture at room temperature for 5 to 30 minutes (time varies according to specimen) for clearing the sample and digesting cellular debris.
* Incubate sputum, pus, CSF, urine, and thin pus smear for 5 to 10 minutes.
* Incubate skin scrapings, thick swabs, thick pus, and tissue samples for about 20 minutes.
* Incubate hair and nail clippings for 30 minutes.
Note: Heating the solution over a flame or heating block or incubating at 300C will accelerate the digestion process. But, DO NOT LET THE KOH BOIL or DRY OUT. To avoid such cases, place the glass slide over moistened filter paper on a petri plate.
- Examine under a compound light microscope; first at low power (100 X magnification), then shift to high power (400 X magnification). (No need to magnify 1000 times.)
- Observe the fungal morphology, arrangement of fungal cells/hyphae, morphology and arrangement of fungal spores, and in the case of hair specimen, examine the location of fungi in hairs.
b. Modified KOH Mount Methods
KOH mount are modified for better contrast and coloring. Now, following modified KOH mount tests are preferred for better visualization:
- KOH-Calcofluor Mount:
In this modified test, all the procedures and requirements are the same, but the reagent is changed from 10 to 30% KOH to 10 to 30% KOH with 0.01% calcofluor-white (a fluorescent blue dye that binds with cellulose and chitin of fungal components) for sample staining.
Preparation of 20% KOH with 0.01% calcofluor white (100 mL)
- Add 20 grams of KOH pellet in 80 mL of distilled water and shake well to dissolve completely.
- In the solution, add 0.1 grams of calcofluor white powder and stir for the complete dissolution of calcofluor crystals.
- KOH-DMSO Mount
In this modified test, all the procedures and requirements are the same, but the reagent is changed from 10 to 30% KOH to 20% KOH in 40% DMSO (dimethyl sulfoxide) in distilled water for better clearing of the specimen during visualization.
Preparation of KOH-DMSO Reagent (100 mL)
- Add 60 grams of DMSO in 90 mL of distilled water to make a 40% DMSO solution.
- In 80 mL of 40% DMSO solution, add 20 grams of KOH pellet and dissolve completely by stirring.
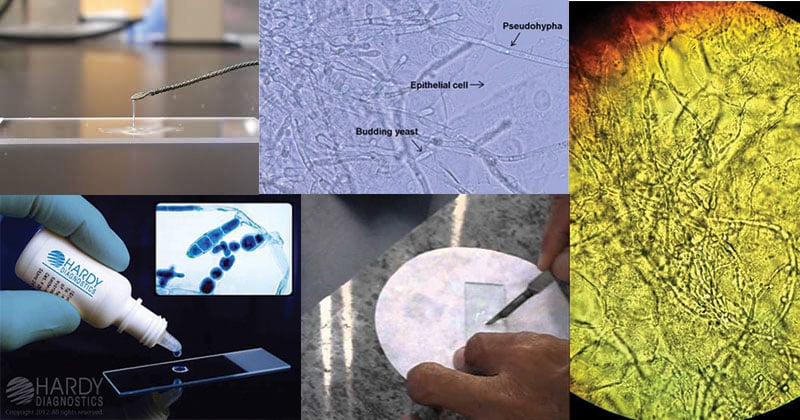
Result and Interpretation of KOH Test
Note the presence of
a) yeast cells
b) pseudohyphae
c) fungal mycelium
d) branching of mycelium
e) budding of yeasts
f) septum in mycelium
g) morphology (projections) in yeast cells
h) structure and arrangement of spores
i) presence of lesions in hair and fungal spores within or outside the hair shaft
Suggested Reports:
- No fungus seen
- Presence of Yeast cells
- Yeast cells with pseudohyphae seen
- Encapsulated yeast cells seen
- Mycelium seen
- Ribbon-like aseptate mycelia seen
- Septate fungal mycelia seen
- Septate hyphae with reproductive structures/dichotomous ~450 branchings (suggesting Aspergillus spp.)
- Positive for pityriasis versicolor (if a combination of round yeast and short hyphae forming the so-called spaghetti and meatball appearance is seen)
- Endothrix or Ectothrix in the hair shaft.
Precautions
- Let the cellular debris be digested completely before observation.
- While heating KOH, don’t let it boil or dry out
- Use an appropriate concentration of KOH solution
- Examine microscope for proper visualization
- Don’t use glass slides with scratches
Applications of KOH Test
- Visualization of fungal morphology
- Confirmation of fungal species in a clinical sample
- Preliminary diagnosis of mycoses to select and start appropriate antimicrobial therapy
- Identification of pityriasis versicolor
- Differentiation of yeasts and filamentous fungus
Advantages of KOH Test
- Easy to perform and rapid result generating
- Don’t need complex and expensive materials/reagents and skills
- Safe to perform
- Suitable for all clinical specimens
Disadvantages/Limitations of KOH Test
- It is a presumptive identification technique only; therefore, we can’t exactly identify the fungal species.
- KOH doesn’t provide color and contrast to the fungal components.
- Require a skilled person to identify/presume fungal genera.
- Some complex tissue materials may require a long time to dissolve or may not dissolve and cause a problem during the visualization of fungi.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- KOH Wet Mount Preparation: Introduction, Principle, Procedure, Result (universe84a.com)
- Reid A. Waldman, Jane M. Grant-Kels,3 – Body dermatitis,Editor(s): Reid A. Waldman, Jane M. Grant-Kels,Dermatology for the Primary Care Provider,Elsevier,2022,Pages 56-111,ISBN 9780323712361,https://doi.org/10.1016/B978-0-323-71236-1.00012-9. (https://www.sciencedirect.com/science/article/pii/B9780323712361000129)
- KOH Mount: Principle, Procedure, Results, Uses • Microbe Online
- KOH Test | definition of KOH Test by Medical dictionary (thefreedictionary.com)
- KOH Wet Mount Preparation: Introduction, Principle, Test Require (medicallabnotes.com)
- KOH Test: Reasons, Procedure, Preparation and Vaginal Wet Mount (medicalhealthtests.com)
- KOH Prep Test: Uses, Side Effects, Procedure, Results (verywellhealth.com)
- Laboratory tests for fungal infections | DermNet (dermnetnz.org)
- KOH Fungal Smear Test: Procedure, Purpose, Results, Cost, Price, Online booking (myupchar.com)

How does your lab perform Quality Control for accurate fungus identification ?
What do you use as a control for accurately identify a fungus microscopically.
How do you adjust the microscope stage and condenser for the best contrast in reading the KOH prep?
How do you prepare for a CLIA inspection of KOH testing?
I would appreciate your help
Great just keep it up.
I need also how to do skin smear for leaprosy.