Interesting Science Videos
Bial’s Test Definition
Bial’s test is a chemical test performed to detect the presence of pentoses and pentosans (derivatives of pentoses). A derivation of this test termed the Bial’s Orchintest is performed to detect the presence of RNA in solutions.
Objectives of Bial’s Test
- To detect the presence of carbohydrates.
- To distinguish the pentoses and pentosans from other derivatives of carbohydrates like the hexoses.
Principle of Bial’s Test
This test is based on the principle that under hydrolysis pentosans are hydrolyzed into pentoses. Further, pentoses are dehydrated to yield furfural, which in turn condense with orcinol to form a blue-green precipitate. In the presence of hexoses, hydroxyfurfural is formed instead of furfural which upon condensation with orcinol forms a muddy brown colored precipitate. The intensity of the precipitation is directly proportional to the concentration of the pentoses in the sample. The intensity of the color developed depends on the concentration of HCl, ferric chloride, orcinol, and the duration of boiling. The concentration of the sugars is determined by measuring the absorbance of 620 nm wavelength in a spectrophotometer or in a red filter colorimeter.
Reaction
Image Source: Harper College.
Requirements
A. Materials required
Equipment:
- UV Spectrophotometer
- Vortex mixer
- Mantle heater/Water Bath.
Chemicals/Reagents:
- Bial’s Reagent
- Ribose sugar
- Other carbohydrates if desired
- Sample
Glasswares and other equipment:
- Test tubes, Test tube stand, Pipettes, Beaker, Ice Test tube caps, Tissue paper, Wash bottle.
B. Reagents
- Bial’s Reagent: 300 mg of orcinol is dissolved in 5 ml ethanol. Add 3.5 ml of this mixture to 100ml of 0.1% solution of FeCL3.6H2O. The reagent thus formed is to be stored in a dark bottle and used within a couple of hours.
- Ribose stock solution: 200µg ribose per mL distilled water stock solution of ribose is to be prepared from the stocked solution. Note: Other carbohydrates of the same concentration can be used as samples if desired. If RNA is used, 300 µg/ml of RNA stock solution is added to Tris-EDTA buffer.
Procedure of Bial’s Test
- Pipette out different volumes (50 µl, 100 µl, and so on) of ribose solution from the supplied stock solution (200µg /ml) into a series of test tubes and make up the volume to 1 mL with distilled water.
- Take a tube labeled as one as blank containing 1ml of just distilled water and the rest of the tubes labeled 2 to 9 for construction of a standard curve. Tubes 10-15 are for the unknown samples.
- Add 5 ml of the bial’s reagent to each tube and mix well by vortexing.
- Cool the tubes.
- Cover the tubes with caps on top and incubate at 90°C for 17 minutes or boiling water bath for 10 minutes.
- Cool the tubes to room temperature and measure the optical density of the solutions at 620 nm against a blank.
- Prepare a standard curve of absorbance against ribose concentration.
- Determine the amount of ribose in the unknown sample by plotting a standard curve of A620 on the Y-axis and concentration of Ribose on the X-axis.
Result and Interpretation of Bial’s Test
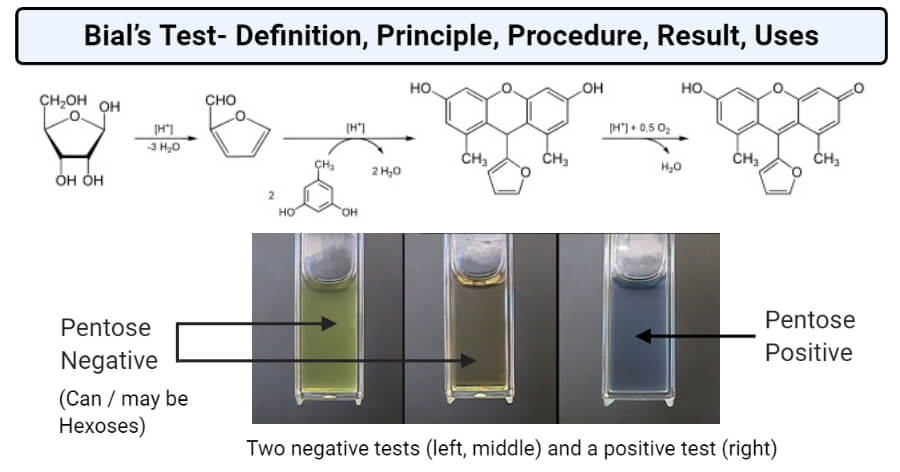
Image Source: Harper College.
- The presence of a blue-green complex indicates the presence of pentoses in the sample.
- Using the graph, the concentration of ribose sugar in the sample can be determined. A similar interpretation can be made for RNA detection as well.
Uses of Bial’s Test
- This test is used to detect the presence of pentose and pentosans in different samples.
- This test can additionally be used for the quantification of RNA in a sample.
Limitations of Bial’s Test
- On prolonged heating, glucoronates might also give a blue-green colored precipitate which might result in false-positive results.
- The color produced might be different with different sugars, and the concentration might not be proportional to the intensity at higher levels.
References and Sources
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- 3% – https://www.iitg.ac.in/biotech/MTechLabProtocols/Carbohydrate%20estimation%20by%20Anthrone.pdf
- 2% – https://en.wikipedia.org/wiki/Biuret_test
- 1% – https://www.slideshare.net/memijecruz/qualitative-analysis-of-carbohydrates
- 1% – https://www.graphpad.com/support/faq/prism-3-calculating-unknown-concentrations-using-a-standard-curve/
- 1% – https://msu.edu/course/lbs/145/luckie/Lab1.html
- 1% – https://en.wikipedia.org/wiki/Bial%27s_test
- <1% – https://www.enzolifesciences.com/science-center/technotes/2017/march/what-are-the-differences-between-pcr-rt-pcr-qpcr-and-rt-qpcr?/
