Monomers are simple, low molecular weight hydrocarbon molecules with two or more binding sites that form covalent linkages with other monomer molecules to form complex structures called macromolecules or polymers. They are the basic units of a polymer.
- The word “monomer” is made up of the prefix mono- means “one,” and the suffix mer- means “part.” The smallest repeating unit is known as a monomer.
- A process by which monomers bind to other monomers by sharing pairs of electrons to form repeating chain molecules is known as polymerization. Two separate molecules bind together, forming a covalent bond. There are two types of polymerization reactions: step reactions and chain reactions.
- The monomer can combine to form dimers (two monomer units), trimers (three monomer units), Tetramer (Four monomer units), Pentamer (Five monomer units), Hexamer (Six monomer units), Heptamer (Seven monomer units), and so on.
- Monomers can originate from synthetic or natural sources.
- Chemical bonds hold the monomer units of a polymer together and maintain the final polymer’s configuration.
- Depending on the monomer units that are binding together, a polymer can have a variety of structures.
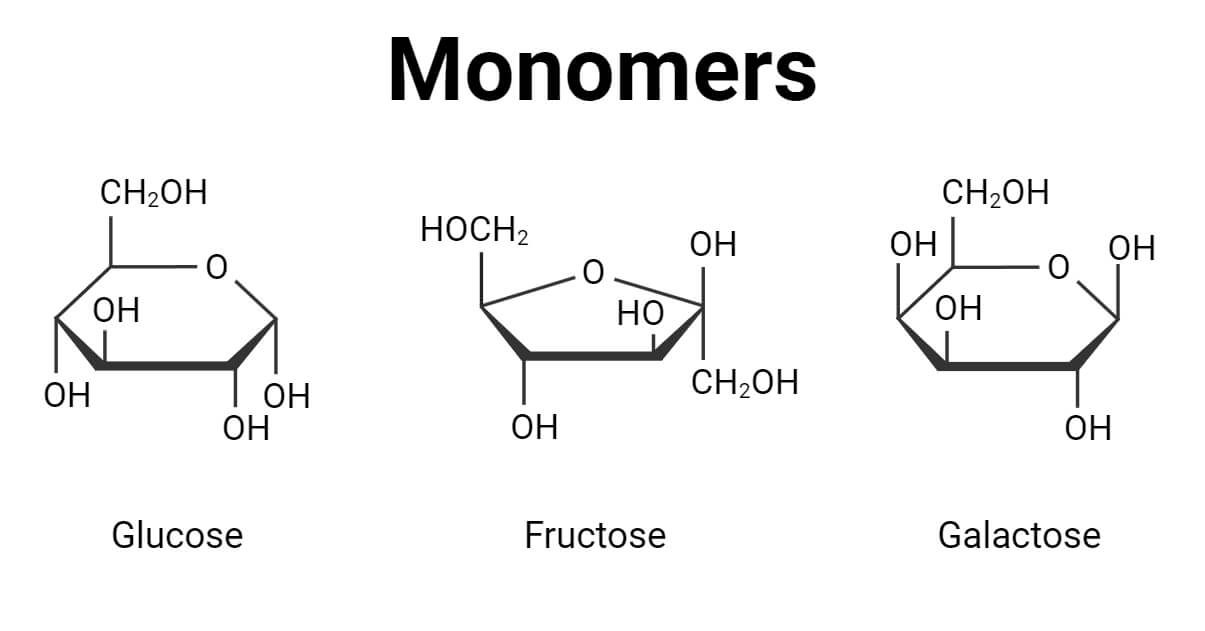
- Many monomer units have varying configurations but the same number of carbon atoms. Isomers are molecules that have the same number of carbon atoms but different configurations.
- The most common examples of isomeric monomers are Glucose, Fructose, and Galactose, which have the same number of atoms in the molecule but different molecular arrangements.
Interesting Science Videos
Types of Monomers
There are three types of monomers. They are as follows:
- Synthetic monomers (Artificially created by combining two different atoms): They are packaging materials, binders’ epoxies, etc.
- Biopolymer (Natural polymer found in living organisms): Three classes of biopolymers are polypeptides and proteins, polynucleotides, and polysaccharides)
- Natural Polymer (Building blocks of biopolymers found in nature)
- Monosaccharides
- Amino acids
- Nucleotides
- Fatty acids
- Isoprene
- Monomers are building blocks for biological macromolecules such as DNA, RNA, protein, and Carbohydrate.
- After the polymers are broken down into their monomeric components at the end of digestion, the body absorbs the monomeric components.
- Likewise, DNA and RNA break into nucleotides, proteins break into constituent amino acids, and carbohydrates break into monosaccharides by enzyme digestion.
- Moreover, these nutrients are utilized to make polymer based on the genetic composition and the body’s instructions.
Example of Monomers
The examples of monomers are Alkenes, vinyl chloride, styrene, acrylonitrile, adipic acid, glycol, etc. Examples of monomers include:
Example 1. One example of a biological monomer is monosaccharide molecules, the most accessible form of energy, which link together to form carbohydrate polymer. Through the process of glycolysis, carbohydrates break and turn into energy form.
Glucose or fructose molecules are the monomers of carbohydrates. In the case of glucose, glycosidic bonds may link sugar monomers to form such polymers as glycogen, starch, and cellulose.
- Monosaccharide is a simple sugar. Glucose, fructose, and maltose are the examples.
- Disaccharides are two monosaccharides bonded together. Sucrose is an example.
- Polysaccharides, a complex sugar, are linked by two or many monosaccharides. Cellulose (wood, paper), starch (energy storage in plants), and glycogen (energy storage in animals) are examples.
Example 2. Amino acid is the monomer of protein. They are linked together by peptide bonds. A peptide bond is a covalent bond that forms between two amino acids during dehydration synthesis.
There are 20 amino acid monomers, but they all have a general structure of C, NH3, COO-, H, and the R group. Polymers join together by polypeptide. Proteins include the collagen found in the skin dermis, bones, and the meninges that cover the brain and spinal cord, as well as the keratin in the skin epidermis that shields beneath structures.
Example 3. Glycerol, a naturally occurring sweet alcohol, and fatty acids, building blocks of fat, are the monomer of lipids. In contrast to monosaccharides, fatty acids cannot be directly metabolized to provide energy.
It requires three processes:
- In the first stage, the body metabolizes fats that accumulated in the body’s Adipose tissue (Lipolysis),
- It goes under activation (they proceed to peroxisomes and the mitochondria),
- These organelles separate the fatty acids by oxidizing the fatty substances,
Diglycerides and triglycerides are the polymers of lipids.
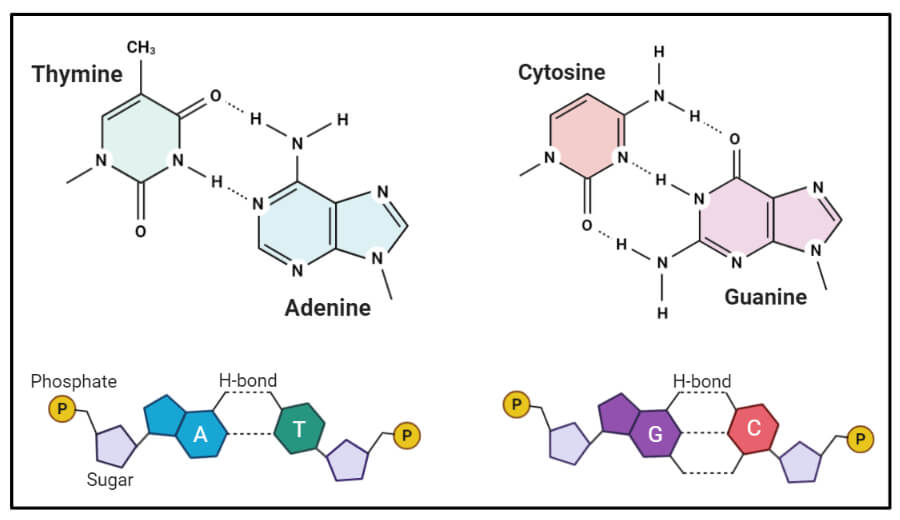
Example 4. Nucleotides are the monomers of nucleic acids. A nucleotide is a crucial organic compound of human structure and function, which is composed of three subunits:
- one or more phosphate groups
- a pentose sugar: either deoxyribose or ribose
- a nitrogen-containing base: adenine, cytosine, guanine, thymine, or uracil
Nucleotides combine to form nucleic acids (DNA or RNA) or the energy compound adenosine triphosphate. Deoxynucleotides are the monomer units of DNA. Polynucleotides are long polymers composed of linear arrays of monomers named nucleotides, which are connected to sugar phosphate by nitrogen bases such as purines and pyrimidines.
Example 5. Isoprene is another natural monomer. Natural rubber, produced by plants, is a polymer of isoprene. Many animals, plants, and also humans can produce isoprene. The body uses the respiratory tract to exhale isoprene.
Classification of Monomers
Monomers are classified based on origin and synthesis:
Classification based on origin
Starches are polymers of monomer glucose, which occur in long chains called amylose or branched chains called amylopectin. They are both stored in plant-based foods and are relatively easy to digest.
Cellulose, a polysaccharide, is a polymer of monomer glucose. It is the primary component of the cell wall of green plants, made from the glucose produced during photosynthesis in plants. It supports a healthy digestive system and promotes a diet high in fiber, which may lower the risk of heart disease and several types of cancer.
Protein is a result of the polymerization of monomer o-amino acids.
Synthetic polymers are man-made polymers. Polythene, polystyrene, PVC, nylon, and dacron are examples.
Classification based on synthesis
The process of repeatedly adding monomers to a polymer chain is called addition or chain polymers. The monomers have a high molecular weight and are unsaturated compounds.
| S.N. | Monomer | Polymer | Uses |
| 1. | CH2=CH2 Ethylene | ~CH2CH2CH2CH2CH2CH2~ Polythene | plastic bags, bottles, toys, electrical insulation |
| 2. | CH2=CHCl Vinyl Chloride | Polyvinyl chloride | bags for intravenous solutions, pipes, tubing, floor coverings |
| 3. | CH2=CHCH3 Propylene | Polypropylene | carpeting, bottles, luggage, exercise clothing |
| 4. | CF2=CF2 Tetrafluoroethylene | ~CF2CF2CF2CF2CF2CF2~ Polytetrafluoroethylene | nonstick coatings, electrical insulation |
| Others monomers and polymer are Butadiene and Polybutadiene. | |||
References
- https://byjus.com/chemistry/monomers/
- https://www.thoughtco.com/definition-of-monomer-605375
- https://biologydictionary.net/monomer/
- https://www.biologyonline.com/dictionary/monomer
- https://web.pdx.edu/~mhutson/345U/lectures/lab4-part2-monomers.pdf
- https://www.scienceabc.com/pure-sciences/what-are-monomers-and-polymers.html
- https://science.howstuffworks.com/monomer.htm
- https://openstax.org/books/anatomy-and-physiology-2e/pages/2-5-organic-compounds-essential-to-human-functioning
- https://www.livescience.com/60682-polymers.html
- https://www.thoughtco.com/amino-acid-373556

Nice noted prativia… Success in your work.. Am a student in school of medicine moi university Kenya am getting help from your site