Molisch test is a group test for all carbohydrates, either free or bound to proteins or lipids. It is a sensitive test that requires precision for the detection of carbohydrates.
Objectives of Molisch Test
- To detect the presence of carbohydrates in a given sample.
- To distinguish carbohydrates from other biomolecules.
Interesting Science Videos
Principle of Molisch Test
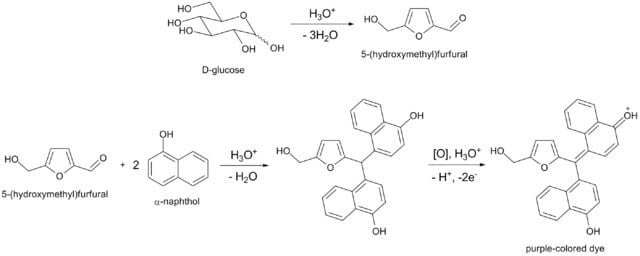
Image Source: AlexNB.
- The reaction is based on the fact that the concentrated acid catalyzes the dehydration of sugars to form furfural (from pentoses) or hydroxymethylfurfural (from hexoses).
- Either of these aldehydes condenses with two molecules of naphthol to form a purple or violet colored complex at the interface of the acid and test layer.
- If the carbohydrate is poly- or disaccharide, a glycoprotein or glycolipid, the acid first hydrolyses it into component monosaccharides, which get dehydrated to form furfural or its derivatives.
- A green ring might be observed if any impurities are present in the reagent as they might interact with the α-naphthol and the acid.
- A rind ring is seen if a concentrated sugar solution is used. This might be due to the charring of the sugar due to the acid.
Requirements
1. Reagent
- Molisch reagent: Dissolve 3.75 g of α-naphthol in 25 ml of Ethanol 99%. This reagent should be prepared fresh.
- Concentrated sulphuric acid
- Test sample
2. Materials required
- Test tubes
- Test tube stand
- Pipette
- Distilled water
Procedure of Molisch Test
- Take 2 ml of each distilled water and test sugar solutions in four test tubes separately.
- Add two drops of Molisch reagent to each tube.
- Hold the test tube in an inclined position and gently add 1 ml concentrated H2SO4 along the wall of the test tube. Do not mix the acid with the solution. A black ring may form if concentrated acid is not added slowly as the heat generated from the reaction can char the carbohydrates.
- Observe the test tube for the formation of a purple-colored ring at the layer between the solution and the acid.
Result and Interpretation of Molisch Test
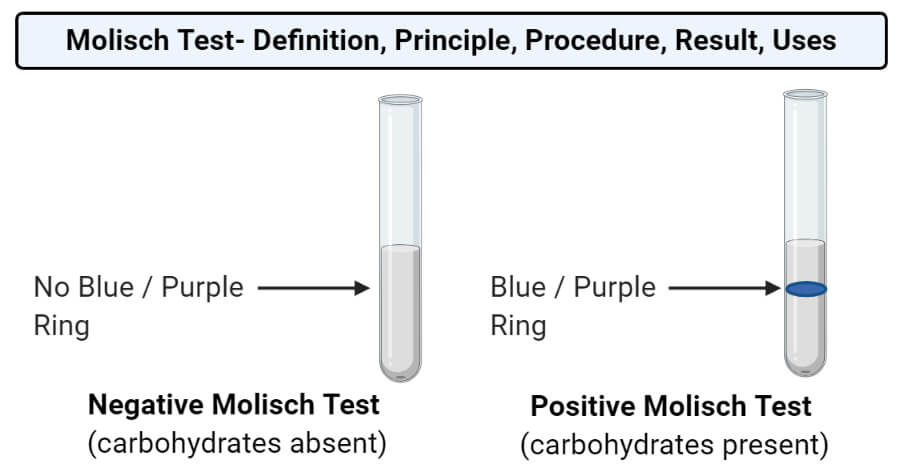
- The formation of the purple colored ring occurs at the interface between the sulphuric acid and the test solution.
- The sulphuric acid remains above the test solution as the acid is denser than the test solution.
- The absence of color indicates a negative result.
Uses of Molisch Test
- Molisch test is used to detect the presence of carbohydrates in different samples.
- It can be used to detect the formation of carbohydrates as a by-product in different reactions and distinguish it from other biomolecules.
Limitations of Molisch Test
- Trioses and tetroses do not have the necessary five carbon atoms for furfural formation, so they do not give a positive result for this reaction.
- Molisch test is not a specific test for carbohydrates. Furfurals as such or furfural yielding substance, some organic acids like citric acids, lactic acid, oxalic acid, formic acid, etc. can give a positive result.
References and Sources
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- 17% – https://www.researchgate.net/profile/Anand_Tiwari_Phd/publication/313745155_Practical_Biochemistry_A_Student_Companion/links/58ab15f04585150402034e42/Practical-Biochemistry-A-Student-Companion.pdf
- 1% – https://www.coursehero.com/file/62741495/54796013-Aldehydesdoc/
- 1% – https://www.biologydiscussion.com/carbohydrates/carbohydrates-monosaccharides-disaccharides-and-polysaccharides/16893
- 1% – https://msu.edu/course/lbs/145/luckie/Lab1.html
- 1% – https://en.wikipedia.org/wiki/Molisch%27s_test
- 1% – http://vlab.amrita.edu/?sub=3&brch=63&sim=1094&cnt=1
- 1% – http://downloads.hindawi.com/journals/ijce/2012/674761.xml
- 1% – http://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/carbo/molisch/molisch.htm
