Interesting Science Videos
Heller’s Test Definition
Heller’s test is a biochemical test performed to detect proteins in a sample by the denaturation of those proteins by the addition of strong acids. Heller’s test usually uses concentrated nitric acid for the denaturation of proteins. The test is performed for clinical purposes to detect abnormal proteins in biological fluids, including urine. Heller’s test is a type of precipitation test where the precipitation is brought about by denaturation. The test was discovered and named after the Austrian Chemist, Johann Florian Heller while studying the biochemical nature of urine. The most common proteins that are detected by Heller’s test in urine include albumin and globulin. The test is clinically important as it is simple and requires a minimum reagent. It also detects proteins in biological fluids that represent the pathological features of certain diseases, thus helping in disease diagnosis.
Objectives of Heller’s Test
- To detect the presence of proteins in a given sample.
- To detect proteins in biological fluids like urine and blood.
- To identify albumin and globulin that might be present in urine.
Principle of Heller’s Test
The test is based on the principle of precipitation of proteins, which in this case takes place in the presence of mineral acids like nitric acid. Protein precipitation by acids relies on the changes in the pH of the solution. As all proteins have a defined isoelectric point or pI value, changes in the pH of the solution affect the structure of the protein. The addition of acids to a solution reduces its pH value. As the value decreases, protein molecules become positively charged due to the proton capture by amino groups present in the proteins. In an aqueous state, the hydration sphere that surrounds the protein becomes disrupted due to the charges. The disruption brings about an imbalance in the structure of the protein, resulting in precipitation. The addition of acid to a protein sample causes precipitation of proteins at the point where the acid comes in contact with the protein solution. As a result, a white coagulated ring is formed between the two layers of protein solution and the mineral acid (HNO3).
Requirements
Reagent
- Nitric acid (HNO3)
- Sample
Materials required
- Test tubes
- Test tube stand
- Pipettes
Procedure of Heller’s Test
- In a clean and dry test tube, 2 ml of concentrated nitric acid is taken.
- To this, 2 ml of urine or other sample is added. The sample should be poured from the sidewall of the test tube in an inclined position in order to form a layer of the sample above the nitric acid.
- The test tube is then observed for the formation of a white ring at the junction of the two layers.
Result and Interpretation of Heller’s Test
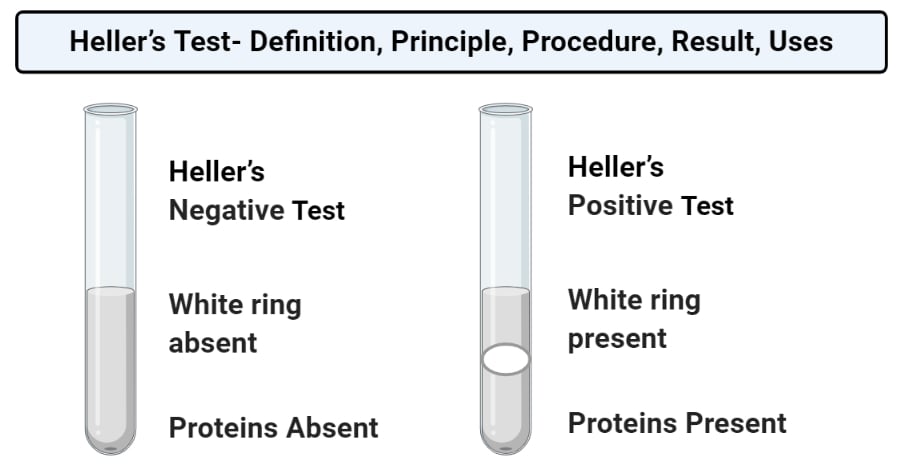
- Positive result: A positive result is represented by the formation of a white ring (precipitated protein) at the junction of the two distinct layers. This indicates the presence of proteins in the given sample.
- Negative result: A negative result is represented by the absence of a white ring. This indicates the absence of protein in the sample.
Uses of Heller’s Test
- Heller’s test is a biological test used for the detection of proteins in biological fluids.
- The test is better than other similar tests as the test requires a small amount of urine sample.
Limitations
- The concentrated acid used in the test is corrosive and thus, should be handled carefully.
- The test is qualitative and doesn’t give the concentration of protein present in the sample.
References
- Nigam S. C. and Omkar (2003). Experimental Animal Physiology and Biochemistry. New Age International Pvt. Limited. New Delhi.
- D (2012). Biochemistry. Fourteenth Edition. Academic Publishers. Kolkata.
- Kyle, R. A., & Shampo, M. A. (1988). Johann Heller and the Nitric Acid Ring Test. Mayo Clinic Proceedings, 63(9), 955.doi:10.1016/s0025-6196(12)62703-7
- https://bioquochem.com/principals-of-various-protein-precipitation-methods/
