Electroporator is a device used in molecular biology and genetic engineering to introduce foreign DNA, RNA, or other molecules into the cells using electric pulses.
Interesting Science Videos
Electroporator History
In 1960, it was discovered that a larger membrane potential is developed at the two poles of a cell when an external electric field is applied. In 1970, it was discovered that the membrane would break and recover as well once membrane potential reaches a critical level. In 1980. Dr. Thomas C. Weaver and his colleagues discovered electroporation by using the same principle. The term electroporation was coined to describe the process which involved electric fields to create transient pores in the cell membrane and allow molecules to enter the cell.
Principle of Electroporation
When the electrical pulse of optimized voltage lasting for a few microseconds is discharged through cell suspension of host cells and selected molecules (e.g., DNA), the phospholipid bilayer of the membrane is disturbed. This leads to the formation of temporary pores. Then the electrical potential across the membrane rises, allowing charged molecules like DNA to enter inside through the pores. The process is similar to electrophoresis.
Types of Electroporation
Basically, there are two main types of electroporation on the basis of cells involved: a) Bulk electroporation b) Single-cell electroporation.
- Bulk electroporation: It involves the electroporation of the bulk population of cells. In this kind of electroporation, a homogeneous electric field is applied to a suspension of cells.
- Single-cell electroporation: It involves the electroporation of individual cells. It has applications in single-cell studies. In this kind of electroporation, an electric field is created locally near a single cell.
Likewise, there are two main types of electroporators on the basis of configuration: a) Open electroporators b) Closed electroporators.
- Open electroporators: In this type of electroporator, users can configure settings like pulse length, pulse intensity, waveform, etc.
- Closed electroporators: In this type of electroporator, the user has to select pre-designed configurations.
Similarly, there are two types of electroporators on the basis of mode of operation: a) Exponential decay electroporators b) Square wave electroporators
- Exponential decay electroporators: These generate electric pulses that have an exponential decay waveform. These pulses can be optimized by differing the voltage and capacitance settings in order to develop a pulse gradient.
- Square wave electroporators: These generate a square wave pulse. These pulses feature the voltage, duration between pulses, frequency of pulses, and time of the pulse.
These two electroporators are most commonly utilized for mammalian electroporation purposes. Besides these, time-constant electroporators are characterized by the time constant pulses which offer a single voltage sustained for a designated period of time.
Electroporator Parts
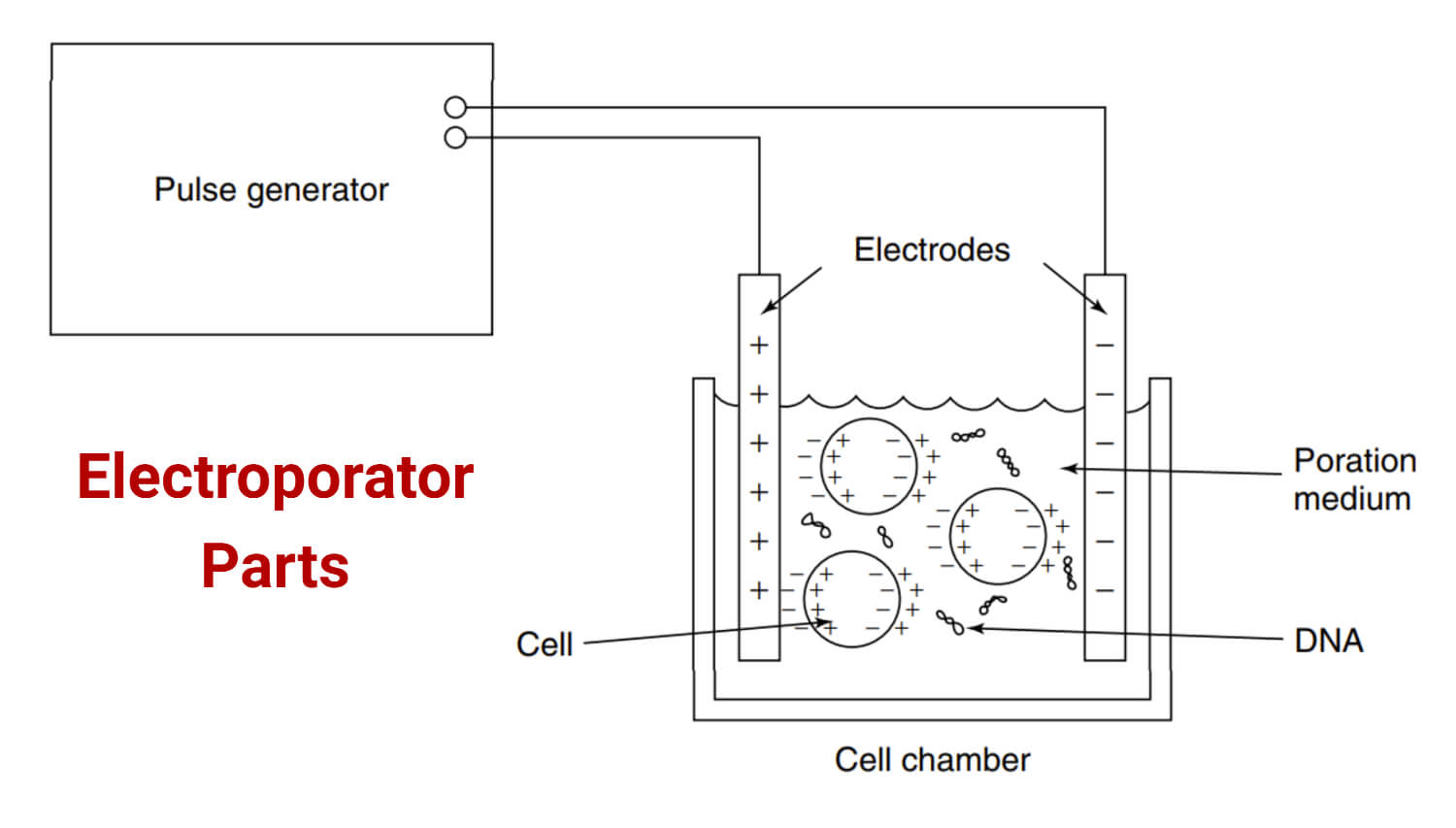
The basic components of an electroporator are:
- Power supply: It is the main component of an electroporator which provides electrical energy to create electrical fields. The voltages offered by the power supply range from a few volts to kilovolts. It can also provide a short pulse of energy.
- Pulse generator: It is the component that controls the amplitude, duration, and frequency of the pulses. Amplitude, duration, and pulse frequency are important parameters for efficient electroporation.
- Cuvettes: They are disposable plastic or glass tubes for holding the cells and molecules. Their size and design might differ to accommodate different sample volumes. They have two metal electrodes on the opposite side which help with electric pulse delivery to the cells.
- Electrodes: These are the components used to deliver the electric pulse to the cells. Proper alignment of electrodes is required to achieve uniform electric field distribution across the cells.
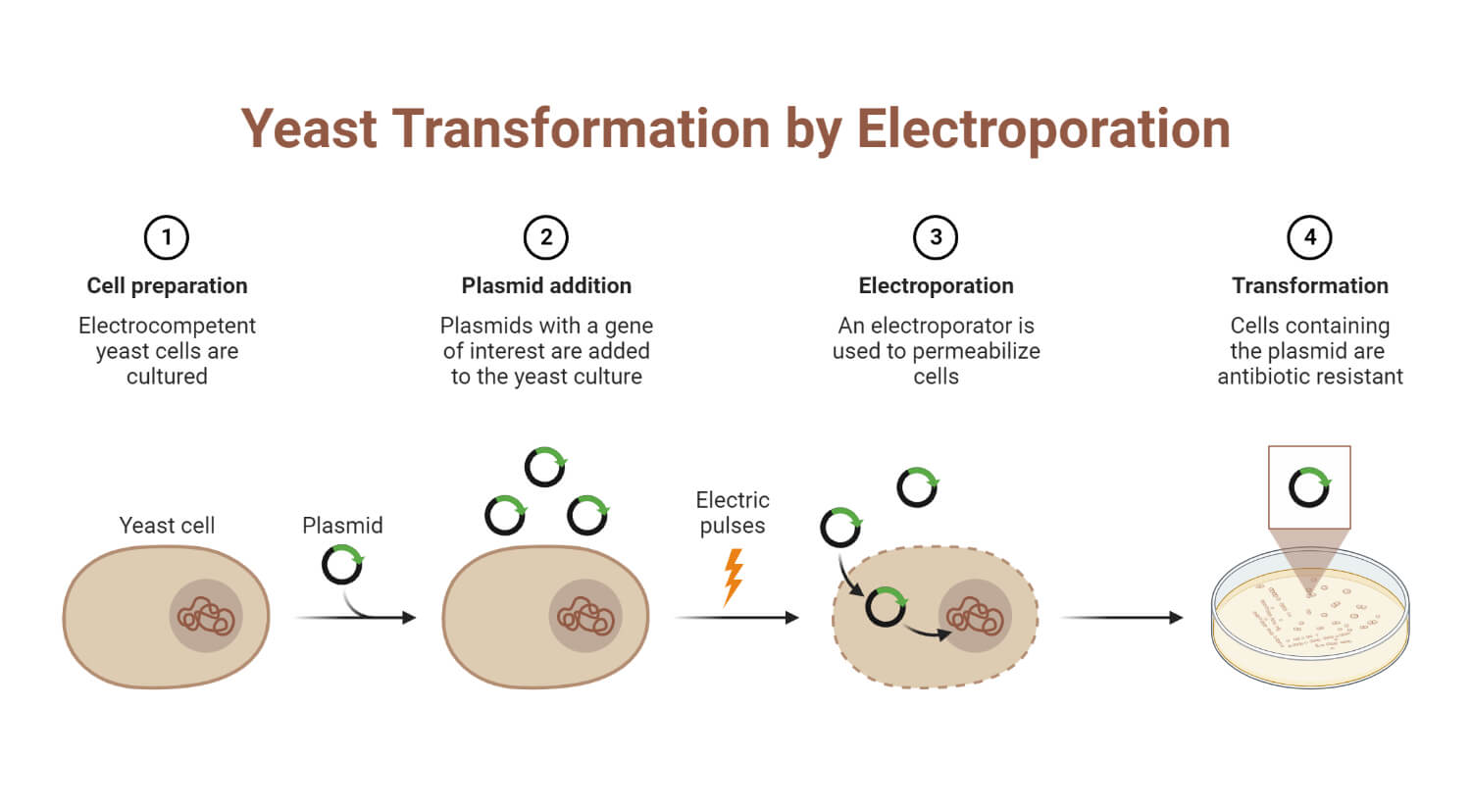
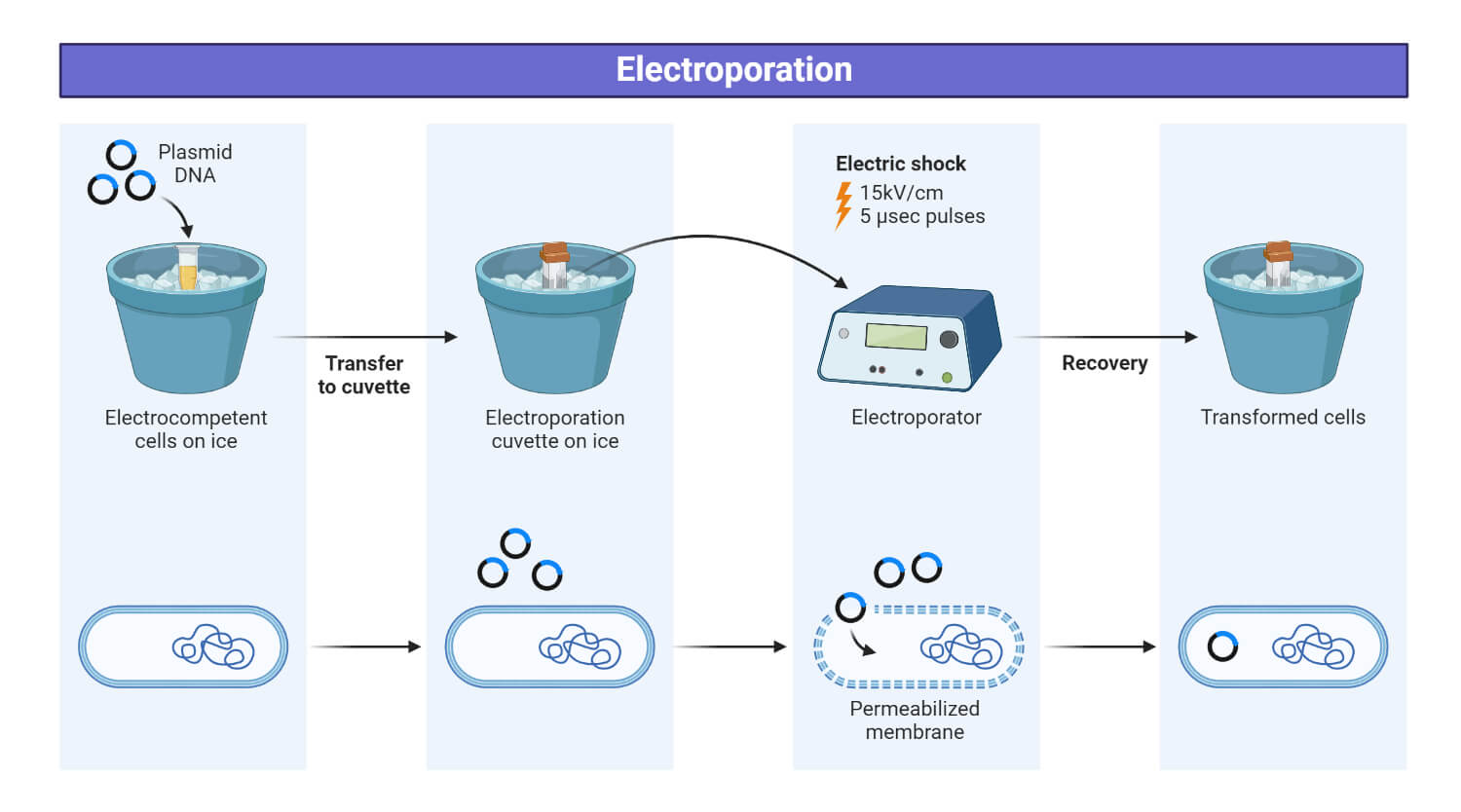
Steps for performing electroporation
Basically, electroporation consists of four steps:
- Cell Preparation: Cells are prepared by suspending them in recommended electroporation buffer
- Electric pulse application: Electric pulse should be applied to cells in the presence of buffer and nucleic acids. In this step, an electric pulse causes potential differences across the cell membrane, so transient pores are created in the membrane, which helps for nucleic acid entry into the cells.
- Cells return to growing conditions: In this step, cells are returned to growth conditions and allowed to recover.
- Cells assay: This is the final step. In this step, cells are assayed for gene expression or silencing.
Applications of Electroporator
- Cell therapy: An electroporator with exceptional cell viability and recovery is used for cell therapy applications.
- Gene therapy: Recent technological advancement has made gene therapy safer and more efficient.
- Cancer treatment: Electrochemotherapy is the best application of electroporation in cancer treatment.
- Genetic Engineering: Electroporation is an easy and simple method for transferring genes/plasmids to bacterial cells.
- Transfection: It is versatile and can be used for the transfection of DNA, RNA, mRNA, RNPs, or proteins in all cell types.
Advantages of Electroporator
- It is time efficient as it takes only a few minutes for transfection or transformation experiments.
- It is versatile as it can be used to introduce foreign components like DNA or RNA or proteins into various types of cells like bacteria, yeast, plant, or animal cells.
- It has high transfection or transformation efficiency.
- It is toxic chemical free, which could damage cells or affect cell viability. It can be used for both large-scale experiments and small-scale experiments. For instance, bulk electroporators are used for large volumes of cell suspensions.
- Electroporators allow users to adjust the electroporation conditions according to need.
- It does not require any vector.
- It is less dependent on cell type.
- Results are reproducible.
- It works well with cell types that are difficult to transfect.
- It is simple and easy to use.
Disadvantages of Electroporator
- It requires parameters to be carefully optimized.
- It can be expensive, especially for an electroporator with advanced features.
- It requires complex setups like electroporators, electrodes, and cuvettes.
- It can have an impact on cell viability if an electrical pulse cause cellular damage.
- Optimizing the electroporation parameters for different cell types can sometimes be time-consuming and challenging.
Precautions while using an Electroporator
- Cuvettes and centrifuges should be pre-chilled in ice before use.
- Electrocompetent cells are needed to be thawed on ice and suspended well.
- Avoid adding large volumes of DNA as it decreases transformation efficiency.
- DNA for transformation should be free from salts and proteins.
- Electroporation should be optimized according to cuvettes and electroporators.
- Avoid high concentrations of salts or air bubbles as they may cause arcing.
- A recovery medium should be added to the cells immediately after electroporation. Delays can decrease the transformation efficiency.
- Petri plates should be pre-warmed at 37°C for 1 hour to expect higher transformation efficiency.
Electroporator Examples
Cell electroporator Eporator® (Eppendorf SE)
- The electroporator allows faster handling of samples using a single button.
- It offers a USB interface for data transfer and GLP for documentation.
- The compact design consumes small space and the safety limit is maximized by the integrated cuvette holder.
- The display indicators allow self-explanatory operation.
Cell electroporator NEPA21 (Bulldog Bio, Inc.)
- It operates at low voltage short-and-long pulses and reversing polarities to deliver perfect square waves with almost the absence of cytotoxic effects.
- The large variety of cells, tissues, and organisms can be transfected with high efficiency.
- It depends on unique non-capacitor-driven pulsing.

Micropulser Electroporator (BioRad)
- The presence of an arc quenching (ARQ) system allows arcing and prevents loss of samples.
- Reproducibility can be maintained by the time constant and volts displayed.
- Wide range of parameters can be optimized manually and the pulse indicators are audible as well as visible.
BTX™ ECM™ 399 Exponential Decay Wave Electroporator (fisherscientific)
- These are portable and user-friendly and feature ECM 399 Generator, PEP™ (Personal Electroporation Pak) Stand (can be ordered separately), and cuvettes and cuvette racks.
- It produces field strength and pulse lengths with precision.
- It also characteristics single-dial control, low and high voltage, 16-character LCD readout, pulse length, and peak voltage feedback.
Gemini Twin Wave Electroporators (BTX)
- A single unit offers both square wave and exponential decay electroporation and is ideal for CRISPR, in vivo, in vitro, in ovo, and other purposes.
- It endorses a system for cuvettes/plates.
- The readily designed protocols are set for the most common eukaryotic and prokaryotic cell types while the user-defined protocols enhance increased ability to add and modify them.
References
- https://international.neb.com/tools-and-resources/usage-guidelines/electroporation-tips
- https://www.thermofisher.com/np/en/home/references/gibco-cell-culture-basics/transfection-basics/methods/electroporation.html
- https://www.future-science.com/doi/pdf/10.2144/btn-2018-2011
- https://www.selectscience.net/products/ecm-830-square-wave-electroporation-system/?prodID=226152
- https://www.mdpi.com/2073-8994/12/3/412
- https://www.medicalexpo.com/prod/eppendorf-se/product-68382-1012911.html
- https://www.medicalexpo.com/prod/bulldog-bio-inc/product-128360-945340.html
- https://www.bio-rad.com/en-np/product/micropulser-electroporator?ID=83527990-34fb-4b33-b955-ca53b57bf8b9
- https://www.fishersci.com/shop/products/btx-harvard-apparatus-ecm-399-exponential-decay-wave-electroporator/BTX399S
- https://www.btxonline.com/gemini-twin-wave-electroporators-2285.html
