One of the fundamental components that make up nucleotides, pyrimidine, is synthesized through a difficult process in living things. Pyrimidine is a crucial part of DNA, RNA, and many other significant biological components.
The process for the de novo synthesis of pyrimidine nucleotides from straightforward precursors is known as the de novo pyrimidine synthesis pathway. The location, route summary, processes involved, significant enzymes and control, salvage pathways, linked illnesses, and the importance of pyrimidine synthesis are only a few of the topics covered on this page.
Interesting Science Videos
Location of De novo pyrimidine synthesis
- In most species, including humans, the de novo pyrimidine synthesis process occurs in the cytoplasm of cells.
- Within the cytoplasm of prokaryotes like bacteria, the complete procedure takes place.
- The first three enzymatic stages of the process take place in the cytoplasm of eukaryotes, which includes animals and plants, whereas the remaining reactions happen in the mitochondria.
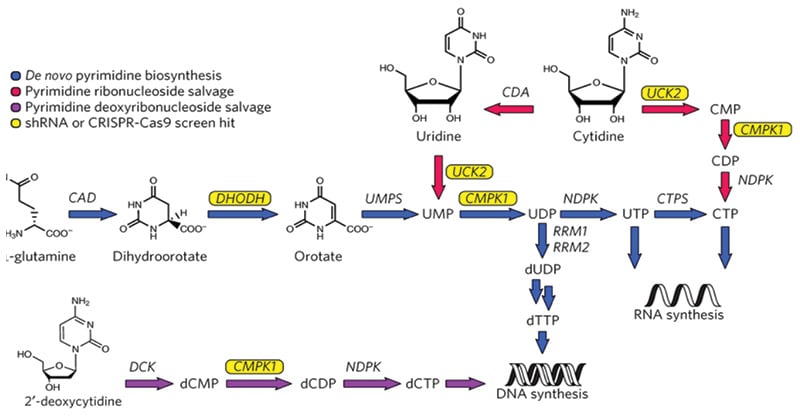
Reactions Involved in De novo pyrimidine synthesis
Simple precursors are transformed into the pyrimidine nucleotides, cytosine, and uracil, by a sequence of enzyme events in the de novo pyrimidine synthesis pathway. There are six main stages in the route, which begin with the activation of bicarbonate and culminate with the creation of orotic acid, which serves as a precursor to pyrimidine nucleotides.
1. Carbamoyl Phosphate Synthesis
- The pathway commences with the conversion of bicarbonate and glutamine into carbamoyl phosphate.
- This reaction is catalyzed by the enzyme carbamoyl phosphate synthetase-II (CPS-II), which requires ATP as an energy source.
- Reaction: Bicarbonate + Glutamine + 2 ATP → Carbamoyl phosphate + Glutamate + 2 ADP + Pi
- Enzyme: Carbamoyl phosphate synthetase-II (CPS-II)
2. Formation of Carbamoyl Aspartate
- In this step, carbamoyl phosphate reacts with aspartate to produce carbamoyl aspartate. The enzyme aspartate transcarbamoylase (ATCase) catalyzes this reaction.
- Reaction: Carbamoyl phosphate + Aspartate → Carbamoyl aspartate + Pi
- Enzyme: Aspartate transcarbamoylase (ATCase)
3. Formation of Dihydroorotate
- Carbamoyl aspartate is then converted into dihydroorotate by the enzyme dihydroorotase.
- This step does not involve the incorporation of any atoms from the other substrates.
- Reaction: Carbamoyl aspartate → Dihydroorotate + H2O
- Enzyme: Dihydroorotase
4. Formation of Orotate
- Dihydroorotate is oxidized to orotate by the enzyme dihydroorotate dehydrogenase. This reaction requires the presence of a flavin mononucleotide (FMN) cofactor.
- Reaction: Dihydroorotate + NAD+ → Orotate + NADH + H+
- Enzyme: Dihydroorotate dehydrogenase
5. Formation of Orotidine 5′-Monophosphate (OMP)
- Orotate is converted into orotidine 5′-monophosphate (OMP) through decarboxylation and the subsequent addition of a phosphate group.
- The enzyme orotate phosphoribosyltransferase (OPRT) catalyzes this reaction.
- Reaction: Orotate + 5-Phosphoribosyl-1-pyrophosphate (PRPP) → OMP + Pyrophosphate
- Enzyme: Orotate phosphoribosyltransferase (OPRT)
6. Conversion of OMP to Uridine 5′-Monophosphate (UMP)
- OMP is finally converted into uridine 5′-monophosphate (UMP) through the addition of a ribose phosphate group.
- This reaction is facilitated by the enzyme orotidine 5′-phosphate decarboxylase (OMP decarboxylase).
- Reaction: OMP → Uridine 5′-monophosphate (UMP) + CO2
- Enzyme: Orotidine 5′-phosphate decarboxylase (OMP decarboxylase)
Important Enzymes and Regulation
De novo pyrimidine synthesis is tightly regulated by a number of important enzymes. ATP and UTP, respectively, act as positive and negative effects on carbamoyl phosphate synthetase-II (CPS-II). ATP and CTP both affect how aspartate transcarbamoylase (ATCase) behaves allosterically. Brequinar, a chemotherapy drug, inhibits dihydroorotate dehydrogenase.
Pyrimidine Synthesis via Salvage Pathways
- Cells contain salvage routes for the production of pyrimidine nucleotides in addition to de novo synthesis.
- Salvage processes reduce the need for de novo synthesis by recycling nucleobases produced during DNA and RNA breakdown.
- Through the use of certain salvage enzymes, the salvage route involves the recovery of free pyrimidine bases such as cytosine, uracil, and thymine.
- The actions of cytidine deaminase and uridine phosphorylase enzymes, respectively, can salvage cytidine and uridine.
- By converting cytidine and uridine into uracil, these enzymes enable the synthesis of pyrimidine nucleotides from scratch.
- The enzyme thymidine kinase, which phosphorylates thymidine to create thymidine monophosphate (TMP), is used in a separate mechanism to salvage thymidine.
De novo pyrimidine synthesis Associated Diseases
Different diseases can result from pyrimidine production dysregulation.
- Orotic aciduria is a rare autosomal recessive illness with poor de novo pyrimidine production that falls under this category.
- Orotic acid builds up in the urine in cases of orotic aciduria due to an enzyme deficiency in either CPS-II or orotate phosphoribosyltransferase (OPRT).
- Anaemia, growth retardation, and anomalies of the urinary system are symptoms. Uridine or uridine triacetate supplements are used as a kind of treatment to get around the pathway’s problematic stage.
- Dihydropyrimidine dehydrogenase (DPD) deficiency is another similar illness. DPD participates in the breakdown of pyrimidine bases.
- Due to their inability to adequately metabolize and remove certain medications, people with DPD deficiency may incur significant toxicity when administered certain chemotherapy medicines, such as 5-fluorouracil.
Significance of Pyrimidine Synthesis
- The basic process of pyrimidine synthesis supplies the essential building blocks for the synthesis of DNA and RNA.
- Pyrimidine nucleotide synthesis needs to be balanced for cells to grow, proliferate, and perform normally as a whole.
- Pyrimidine synthesis disruptions can have serious effects on cellular homeostasis and play a role in the emergence of a number of illnesses.
- It is practical for medicine to comprehend the complexities of the de novo pyrimidine production process.
- Potential treatment approaches for illnesses like cancer might entail focusing on certain enzymes that are part of the process.
- Cancer cells that divide quickly can be prevented from growing and proliferating by blocking the production of nucleotides by inhibiting enzymes such as CPS-II or dihydroorotate dehydrogenase.
Conclusion
The route for de novo pyrimidine synthesis is crucial because it produces the pyrimidine nucleotides required for DNA, RNA, and other essential biological components. In this route, straightforward precursors are transformed into pyrimidine nucleotides by a series of enzyme activities.
The salvage processes use nucleobases from degraded DNA and RNA to supplement de novo synthesis. The importance of maintaining the equilibrium in nucleotide production is highlighted by the fact that dysregulation of pyrimidine synthesis can cause a variety of diseases.
Both fundamental research and the creation of treatment plans for conditions like cancer are impacted by our understanding of the intricate processes involved in pyrimidine production.
Undoubtedly, more research in this area will provide new information on how pyrimidine synthesis is regulated and how important it is to cellular physiology.
References
- Witz, Sandra, et al. “De novo pyrimidine nucleotide synthesis mainly occurs outside of plastids, but a previously undiscovered nucleobase importer provides substrates for the essential salvage pathway in Arabidopsis.” The Plant Cell 24.4 (2012): 1549-1559.
- Schröder, Michael, Norbert Giermann, and Rita Zrenner. “Functional analysis of the pyrimidine de novo synthesis pathway in solanaceous species.” Plant physiology 138.4 (2005): 1926-1938.
- Moffatt, Barbara A., and Hiroshi Ashihara. “Purine and pyrimidine nucleotide synthesis and metabolism.” The Arabidopsis Book/American Society of Plant Biologists 1 (2002).
- Roosild, Tarmo P., et al. “Mechanism of ligand-gated potassium efflux in bacterial pathogens.” Proceedings of the National Academy of Sciences 107.46 (2010): 19784-19789.
- An, Songon, et al. “Reversible compartmentalization of de novo purine biosynthetic complexes in living cells.” Science 320.5872 (2008): 103-106.
- Jones, Mary Ellen. “Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis.” Annual review of biochemistry 49.1 (1980): 253-279.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- https://www.slideshare.net/hirapure/de-novo-and-salvage-pathway-of-purines
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.

I’d like to use the figure ‘De novo pyrimidine synthesis’ in my paper manuscript with the URL ‘https://microbenotes.com/de-novo-pyrimidine-synthesis/’. I will submit it to the American journal ”Pesticide Biochemistry and Physiology’.
I would be so grad and grateful to you if you can kindly give me your permission. Please send back to me.