Pyrimidine is a class of organic heterocyclic compounds containing 2 nitrogen atoms in their carbon ring. Pyrimidines are defined as polyunsaturated six-membered aromatic heterocyclic compounds containing two nitrogen atoms at the 1, 3 – position of the ring.
In the family of heterocyclic compounds, pyrimidine, and its derivatives are of great importance as structural subunits of nucleic acids, vitamins, hormones, antibiotics, and several other biological and artificial compounds.
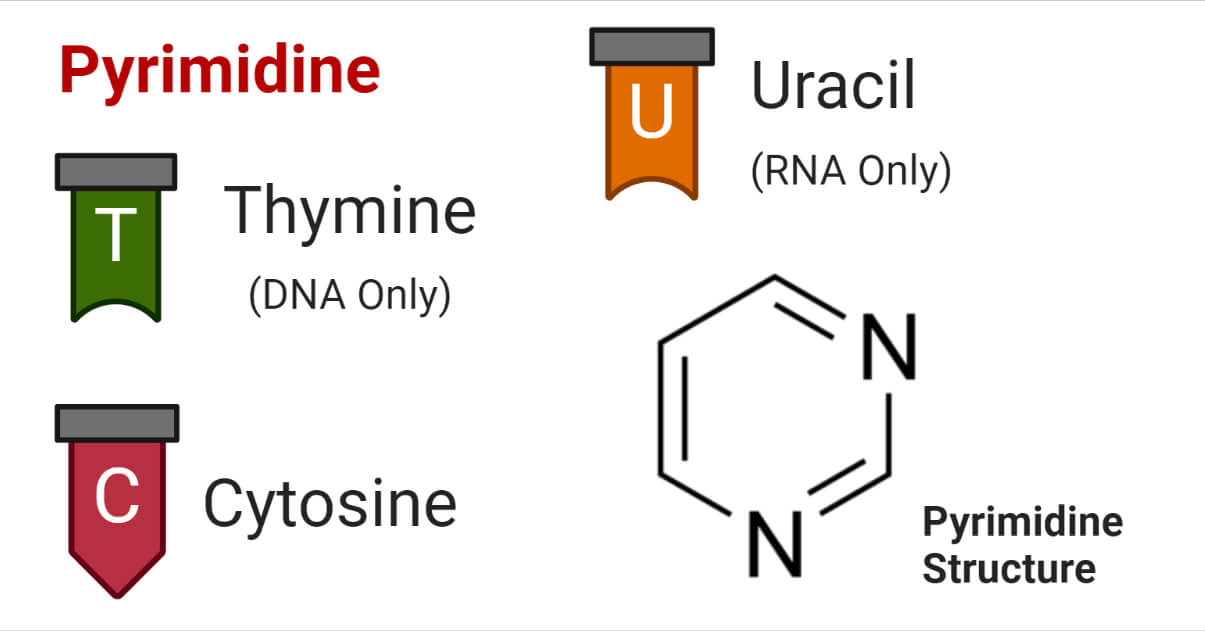
The most known and well-studied pyrimidine derivatives are thymine, cytosine, and uracil; the pyrimidines found in nucleic acids as building blocks. In this article, we will focus on these three pyrimidines.
Interesting Science Videos
General Structure of Pyrimidine Class
Pyrimidine is a cyclic organic compound with a ring of 6 atoms; four carbon atoms with attached hydrogen or side chain and 2 nitrogen atoms at -1, and -3 positions. The carbon (C) and nitrogen (N) atoms are bonded by single and double bonds altering each other.
The N and C atoms are bonded with hydrogen or other functional groups to form different derivatives of pyrimidine.
Thymine
Thymine (abbreviated as T) is a pyrimidine nucleobase of DNA molecules, chemically linked with a deoxyribose sugar, a phosphate group. It binds with an adenine (A) of another strand of the DNA molecule. It is also called ‘5-methyluracil’ and is present in DNA only.
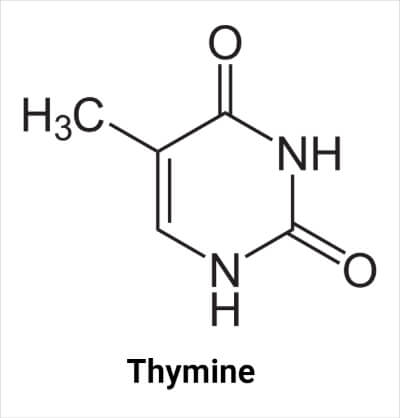
Structure of Thymine
The molecular formula of thymine is C5H6N2O2. It contains two N atoms at the -1 and -3 positions of the ring, each bonded with a hydrogen atom. In nucleic acids, the N atom at the 3- position forms a glycosidic bond with sugar (deoxyribose in DNA and ribose in RNA), resulting in the formation of thymidine. The C atom at the 5- position in nucleic acid is bonded with the methyl group. The carbon at the 2- and 4- positions are bonded with an oxygen atom. There is a double bond between carbon atoms at positions 5- and 6-.
Cytosine
Cytosine (abbreviated as C) is another pyrimidine nucleobase found in both DNA and RNA molecules. It is chemically linked with Guanine (G) of another strand of the DNA. In RNA molecules, it is bound to another nucleobase through phosphate linkage.
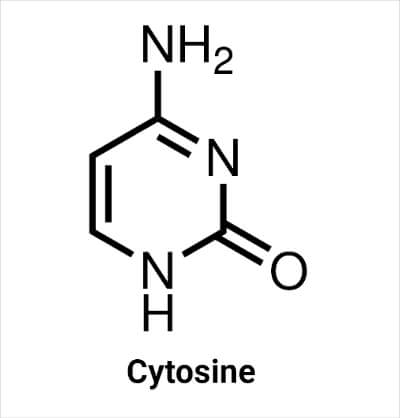
Structure of Cytosine
The molecular formula of cytosine is C4H5N3O. It contains two N atoms at the -1 and -3 positions of the ring and one N atom in the amino group attached with C4 at the 4- position of the ring. In nucleic acids, the N atom at the 1- position forms a glycosidic bond with sugar (deoxyribose in DNA and ribose in RNA), resulting in the formation of cytidine. The carbon at the 2- position is bonded with an oxygen atom, and at the 5- position is bonded with a hydrogen atom. There is a double bond between carbon atoms at positions 5- and 6- and carbon at 4- position and N at 3- position.
Uracil
Uracil (abbreviated as U) is a pyrimidine nucleobase found in RNA molecules only. It is found instead of thymine in RNA. It is also chemically linked to the phosphate group and ribose sugar to form the RNA backbone.
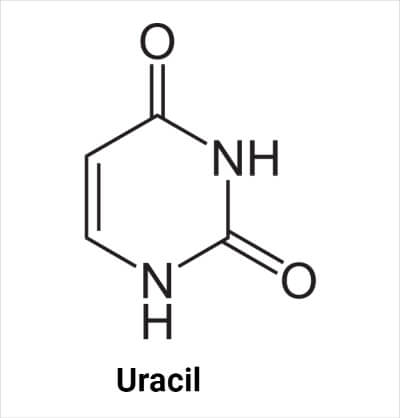
Structure of Uracil
The molecular formula of uracil is C4H4N2O2. Its structure is similar to thymine, but the methyl group at the C atom at the 5- position is replaced by the hydrogen (H) atom.
Derivatives of Pyrimidine
- Thymine
- Uracil
- Cytosine
- Fluorouracil
- Orotic Acid
- Thiamine (Vitamin B1)
- Barbituric Acid
- Veranal
- Antifolate drugs (2-Amino-4-hydroxy pyrimidines)
- Sulfa-drugs
- Antibiotics like disubstituted and trisubstituted pyrimidine contain antibiotics like amicetin, plicacetin, etc.
- Antivirals such as 5-iododeoxyuridine, 5-iodo-2′-deoxyuridine, 1-(3-Azido-2,3-dideoxypentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione, etc.
- Antifungal drugs like fluorinated pyrimidine called flucytosine, hexetidine, etc.
Uses of Pyrimidine
In Medicine
Pyrimidine derivatives are used as anticancer, anti-inflammatory, antibiotic, antiviral, and antifungal agents. Some examples of pyrimidine derivatives used as medicines are listed above.
A Pyrimidine analog, orotic acid, is used in metabolic therapy to prevent heart failure in cardiovascular patients.
As Nitrogen Bases of Nucleic Acids
Thymine, uracil, and cytosine are pyrimidine nitrogen bases of nucleic acids. They form the backbone of nucleic acids. They are also used to prepare nitrogen bases as elements for PCR artificially.
As Subunits For the Production of Vitamins, Toxins, Amino-acids and Other Biomolecules
Several proteins, amino acids, and vitamins contain pyrimidine as their sub-units. Different derivatives of pyrimidine are used in the synthesis of such molecules.
Health Effects of Pyrimidines
- Pyrimidines and their derivatives are building blocks of nucleic acids, vitamins, amino acids, etc. Hence, they help in proper body physiology and development.
- They provide vital nutrients.
- Several derivatives of pyrimidine delays aging and boost immunity.
- Several animal species use pyrimidine derivatives for toxin production.
- Pyrimidine metabolism disorders may result in allergic reactions, encephalopathies, neonatal seizures, etc.
References
- Purines and Pyrimidines – Biology Wise
- Britannica, The Editors of Encyclopaedia. “thymine”. Encyclopedia Britannica, 25 Sep. 2013, https://www.britannica.com/science/thymine. Accessed 17 March 2023.
- Thymine. (2023, January 19). In Wikipedia. https://en.wikipedia.org/wiki/Thymine
- Britannica, The Editors of Encyclopaedia. “cytosine”. Encyclopedia Britannica, 18 Oct. 2007, https://www.britannica.com/science/cytosine. Accessed 17 March 2023.
- Cytosine (genome.gov)
- Pyrimidine – The Definitive Guide | Biology Dictionary
- Britannica, The Editors of Encyclopaedia. “uracil”. Encyclopedia Britannica, 17 Dec. 2018, https://www.britannica.com/science/uracil. Accessed 17 March 2023.
- Uracil (genome.gov)
- Thymine – Definition and Structure | Biology Dictionary
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 1135, Thymine. Retrieved March 17, 2023 from https://pubchem.ncbi.nlm.nih.gov/compound/Thymine.
- Thymine: Definition, Mutation, Structure, Properties and Bases – Scope Heal
- Cytosine Structure – C4H5N3O – Over 100 million chemical compounds | Mol-Instincts (molinstincts.com)
- Cytosine | C4H5N3O – PubChem (nih.gov)
- Cytosine Molecule Structure & Function | What is Cytosine? – Video & Lesson Transcript | Study.com
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 1174, Uracil. Retrieved March 17, 2023 from https://pubchem.ncbi.nlm.nih.gov/compound/Uracil.
- Uracil: Structure, Definition, & Functions I Research Tweet
- Pyrimidine and its derivatives (slideshare.net)
- Mohamed MS, Awad SM, Sayed AI. Synthesis of Certain Pyrimidine Derivatives as Antimicrobial Agents and Anti-Inflammatory Agents. Molecules. 2010; 15(3):1882-1890. https://doi.org/10.3390/molecules15031882
- Pyrimidine – Structure, Uses, Functions and Synthesis (vedantu.com)
- Vinita Sharma, Nitin Chitranshi, Ajay Kumar Agarwal, “Significance and Biological Importance of Pyrimidine in the Microbial World”, International Journal of Medicinal Chemistry, vol. 2014, Article ID 202784, 31 pages, 2014. https://doi.org/10.1155/2014/202784
- Patil, Sharanabasappa. (2018). Biological and Medicinal Significance of Pyrimidine’s: A Review. International Journal of Pharmaceutical Sciences and Research. 9. 44-52.
- Disorders of Purine and Pyrimidine Metabolism – an overview | ScienceDirect Topics
