ATP (Adenosine Triphosphate) is a pyrophosphate molecule that provides energy for conducting metabolic processes, i.e., sustaining the life of a cell.
It is a complex organic high-energy compound that provides energy for conducting metabolic processes. It is referred to as “the molecular unit of currency” of the intracellular energy transfer or “Energy Currency of the Cell” or “energy unit of the cell”. It is the primary energy source for use and storage inside every cell.
It is a complex organic molecule consisting of adenine, ribose, and a triphosphate moiety. The energy released during cellular respiration is trapped in the form of two phosphodiester bonds in the ATP molecule. During the hydrolysis of these high-energy phosphodiester bonds in ATP molecules, energy is released, then used for cellular activities.
IUPAC Name: Adenosine 5′-(tetrahydrogen triphosphate)
Molecular Formula: C10H16N5O13P3
Molecular Weight: 507.18 g/mol
Density: 1.04 g/cm3
Solubility: Water soluble
Interesting Science Videos
Structure of ATP
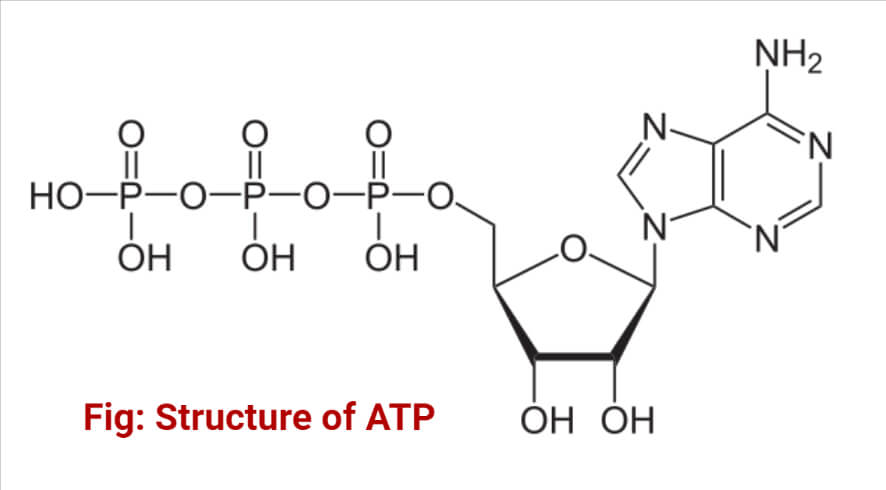
It consists of adenine, ribose, and a triphosphate moiety. Adenosine is attached by the 9-nitrogen atom to the 1-carbon atom of ribose which in turn is attached at the 5-carbon atom of sugar to a triphosphate group. Three phosphate groups form a triphosphate moiety. They are termed alpha (α), beta (β), and gamma (γ) phosphate groups. There are three phosphodiester bonds; one between phosphate groups, the second between the phosphate groups, and the third between the phosphate and ribose sugar. The first two are high-energy phosphodiester linkage and produce energy during hydrolysis. Hence, hydrolysis of ATP to ADP (Adenosine Diphosphate) and again to AMP (Adenosine Monophosphate) yields energy, but the breaking of the phosphodiester bond between ribose and the phosphate requires energy.
Production of ATP
ATP is an energy-rich compound primarily synthesized during cellular respiration in aerobic and anaerobic cells. Oxidation of glucose, lipids (fats), and amino acids produce the ATP molecules inside cells. The energy released during the oxidation of these nutrients is trapped in the form of the high-energy phosphodiester bond in the ATP molecule.
Glucose and ATP
Carbohydrate is the primary source of energy. Carbohydrates consumed in different forms (starch, sucrose, dextrose, lactose, fructose, etc.) are mostly broken down to monosaccharide form ‘glucose.’ Glucose is then subjected to metabolic reactions, glycolysis, Krebs cycle, and oxidative phosphorylation and is oxidized to release energy. This released energy is trapped and stored in the form of ATP.
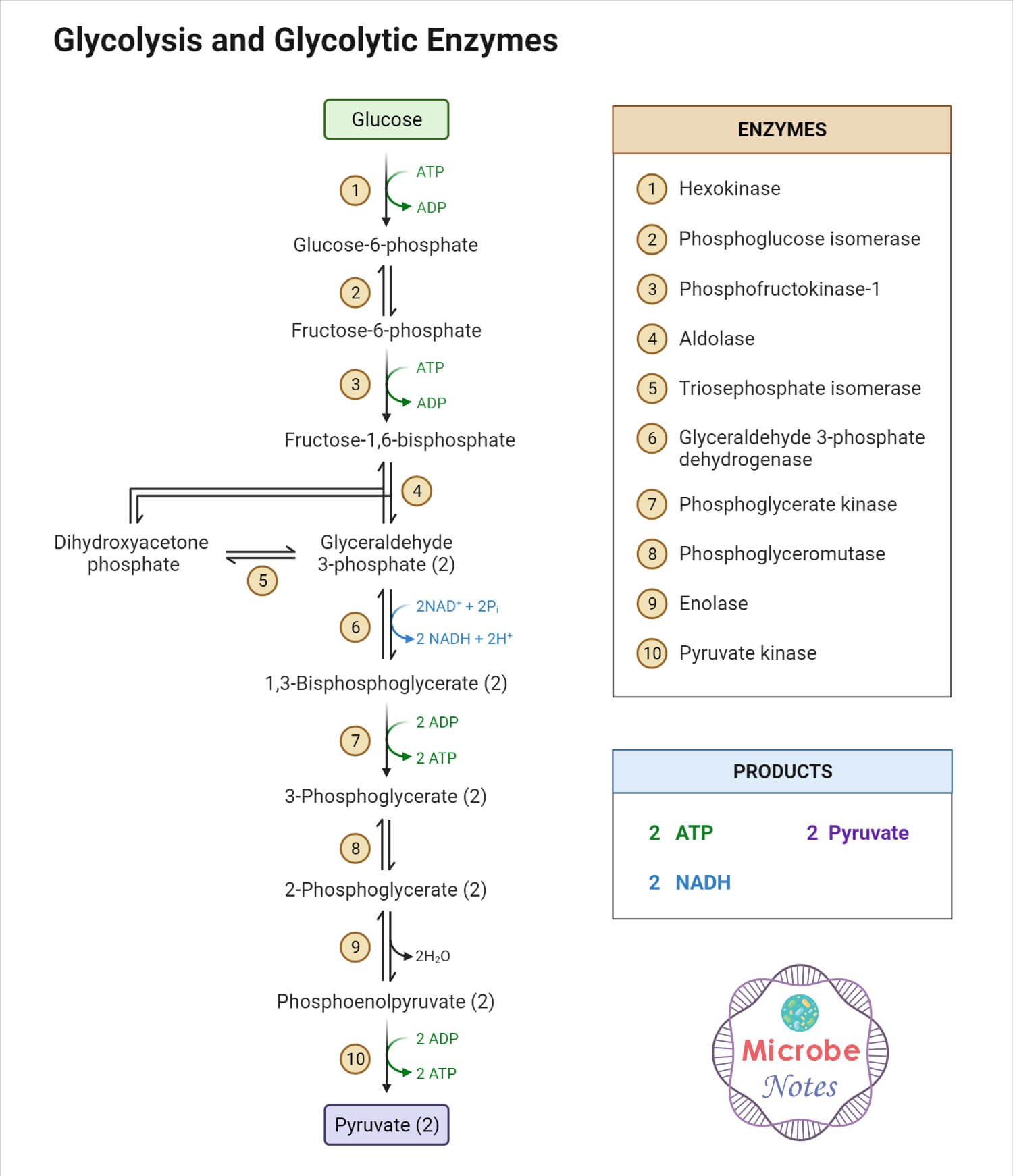
Similarly, proteins and lipids metabolism also produce simple end products like acetyl CoA, succinyl CoA, keto-acids, ammonia, etc., which are then subjected to the Krebs cycle and oxidative phosphorylation to yield ATP molecules.
ATP Synthesis Mechanisms
ATP synthesis occurs during several cellular processes, including phosphorylation reactions. It can occur in both aerobic and anaerobic conditions. The significant ways of ATP production are; cellular respiration (oxidative phosphorylation, substrate-level phosphorylation), beta-oxidation and lipid catabolism, protein catabolism, photo-phosphorylation, and fermentation.
1. Cellular Respiration
It is the process where glucose is catabolized into acetyl – CoA and subjected to oxidative phosphorylation for ATP synthesis. It is the major mechanism for synthesizing most of the ATP required for a cell. The ATP production via cellular respiration occurs in two different stages;
a. Substrate level phosphorylation
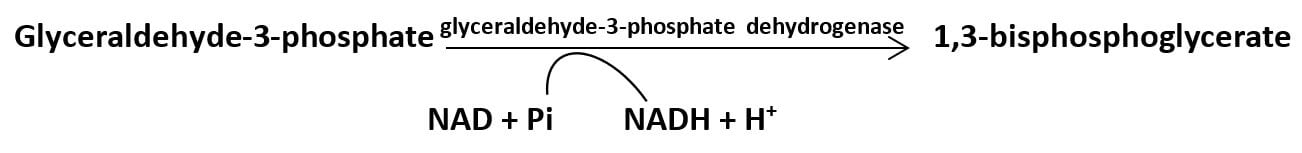
ATP production occurs directly during glycolysis. In the glycolytic pathway, oxidation of G-3-P by G-3-P dehydrogenase enzyme adds a high energy phosphate group which is transferred to ADP in the next reaction generating ATP molecule.
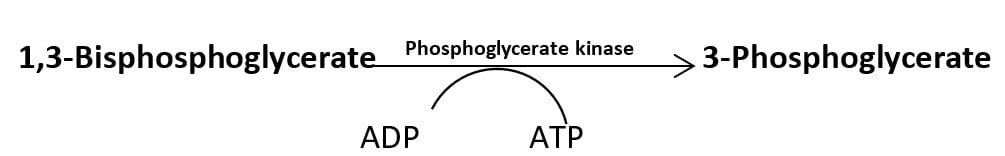
In another reaction, the energy released during dehydration of 2-phosphoglycerate converts the low energy phosphate bond into a high energy phosphate bond which is transferred to ADP in the next reaction producing an ATP molecule.

Pyruvate is then oxidized to acetyl – CoA molecule by pyruvate dehydrogenase complexes. Thus formed acetyl – CoA is then subjected to the Krebs cycle, where it is oxidized to produce one equivalent of ATP, i.e., GTP molecule, three molecules of NADH, and one molecule of FADH2. These NADH and FADH2 molecules are electron carriers that will enter the ETC (electron transport chain) and produce ATP molecules.
b. Oxidative phosphorylation
The intermediate compounds like NADH and FADH2 produced during glycolysis, pyruvate decarboxylation, and Krebs cycle are used as electron carriers and subjected as the substrate to the electron transport chain (ETC) generating proton gradient. The proton gradient is coupled with chemiosmosis, where the ATP synthase enzyme synthesizes ATP.
2. Photo-phosphorylation
It is the process where the light energy is used to phosphorylate ADP to ATP inside chlorophyll-containing cells. The general reaction of photophosphorylation can be expressed as:
ADP + light energy + Pi → ATP
It is of two types; cyclic and non-cyclic photophosphorylation.
a. Cyclic Photo-phosphorylation
It is the photo-phosphorylation process where electrons released by the P700 pigment of Photosystem-I are recycled back to Photosystem-I. The electron released is subjected to an ETC which generates a proton gradient that is used to produce ATP by ATP synthase in a process called chemiosmosis. It predominately occurs in bacterial cells.
b. Non-cyclic Photo-phosphorylation
It is the photo-phosphorylation process where the released electrons are not recycled back to the photosystem which produces them. In this mechanism, both photosystem-I and –II are excited simultaneously. Electrons released by P680 of photosystem-II are passed through an ETC generating ATP by phosphorylation of ADP by ATP synthase enzyme in chemiosmosis. The electrons are then used to replace the electrons lost by P700 of photosystem-II during photoexcitation. The electrons released by photosystem-II are then used to reduce NADP+ to NADPH. It predominately occurs in plant cells and causes the release of one O2 molecule in each step.
3. Beta-oxidation
It is a catabolic reaction where fatty acids are oxidized to acetyl – CoA which are then subjected to the Krebs cycle, and the ETC simultaneously for the generation of ATP. During each beta-oxidation cycle, one acetyl – CoA, NADH, and FADH2 are produced. These intermediate products then further metabolize releasing ATP in the Krebs cycle and oxidative phosphorylation processes.
4. Fermentation
It is the process of production of organic acid or alcohol through the reduction of pyruvate produced by glycolysis of sugar (glucose). It occurs in the anaerobic respiration process. It is a substrate-level phosphorylation process where 2 ATP molecules are produced from a single glucose molecule. The end product is either lactic acid or ethanol. These products can’t enter oxidative phosphorylation due to a lack of oxygen. Hence no further ATP molecules are produced. Therefore, it is less effective than the aerobic respiration process in ATP generation.
Hydrolysis of ATP
It is the catabolic reaction process where the energy-rich phosphodiester bonds of ATP molecules are broken down (hydrolyzed), releasing energy and inorganic phosphate molecules in the presence of water and ATPase enzyme. It is an exergonic reaction where the energy stored in the phosphodiester bond during ATP formation is released. This released energy is used by the cell for performing several cellular activities and reactions.
ATP is first hydrolyzed, breaking one energy-rich phosphodiester bond to form ADP. The ADP molecule can further be hydrolyzed breaking another energy-rich phosphodiester bond to form AMP. The breakdown of phosphodiester bond is catalyzed by ATP hydrolase (ATPase) enzyme in presence of water. ATP hydrolysis is a reversible reaction i.e., ADP and AMP can be rephosphorylated from ATP molecule.
Hydrolysis of ATP to ADP releases 7.3 kCal/mol of energy. It can be expressed as:
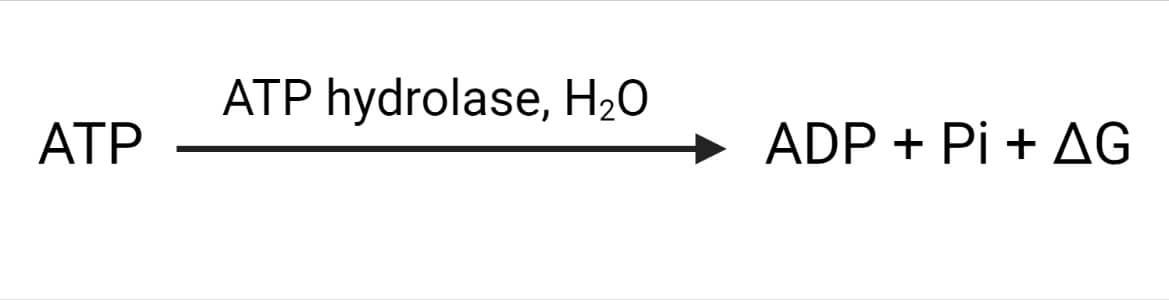
Where, ∆G= Gibbs free energy = – 7.3 kCal/mol energy
Further hydrolysis of ADP to AMP releases 7.5 kCal/mol of energy. It can be expressed as:
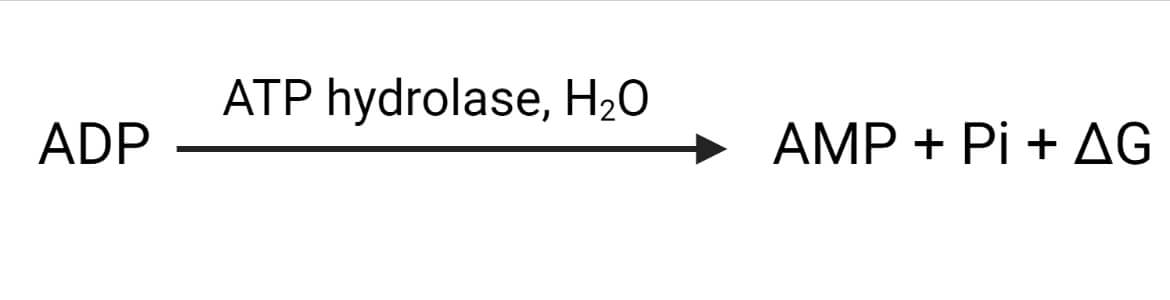
Where, ∆G= Gibbs free energy = – 7.5 kCal/mol energy
The overall reaction can be summarized as:
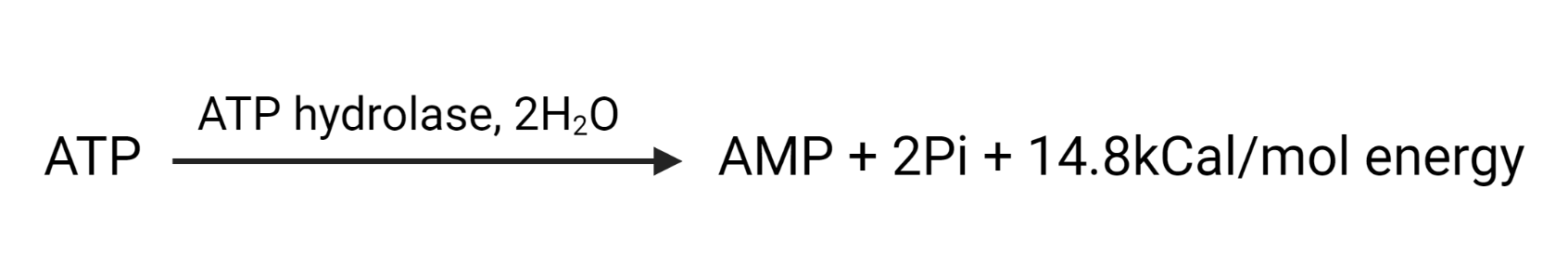
Functions of ATP
- ATP plays a significant role in anabolic reactions by providing energy for bone formation or breaking. It is the primary source of energy for cellular reactions and processes. Energy is stored and transported in the form of ATP inside living cells. All other forms of chemical energy in the cell are converted to ATP before use.
- Vital processes like muscle contraction-relaxation, cellular movements, impulse transmission, heart pumping, blood circulation, etc., require ATP hydrolysis as fuel.
- ATP is used as the energy source for transporting molecules in and out of the cell during active transport mechanisms.
- ATP acts as an intracellular reserved source of energy.
- ATP is involved in intracellular signaling processes. They serve as the substrate for kinases for phosphate transfer, adenylate cyclase enzymes, etc. ATP is converted to cAMP (cyclic AMP), which acts as secondary signaling molecules during intracellular signaling processes.
- ATP is also involved in extracellular signaling and neurotransmission. During the purinergic signaling process, ATP is used for cell-to-cell communication. It also serves as a neurotransmitter in several neural signaling processes.
- ATP is required for the biosynthesis of DNA and RNA molecules. DNA gyrase of prokaryotes or DNA topoisomerase II requires ATP in form of dATP (deoxyribonucleotide adenosine triphosphate).
- It is also involved in protein synthesis reactions by activating aminoacyl – tRNA synthetase enzymes.
- Several ATP binding cassette transporters (ABC transporters) are present in cell membranes which use the energy of ATP binding and hydrolysis for cellular transportation like the uptake of vitamins, metal ions, biosynthetic precursors etc and efflux of lipids, drug residues, sterols, etc.
- Injectable ATPs are used as diagnostic and therapeutic drugs for certain cardiac disorders (cardiac bradyarrhythmias).
- ATP is found to act as a biological hydrotrope. ATP can hinder the thermal aggregation of proteins and the solubility of proteins.
- ATP is also being studied for its anti-aging properties and is used in anti-aging drugs.
References
- Nelson, D. L., & Cox, M. M. (2017). Lehninger principles of biochemistry (7th ed.). W.H. Freeman.
- Satyanarayana, U. (2013). Biochemistry. Elsevier Health Sciences. https://books.google.com.np/books?id=Bd9XAwAAQBAJ
- Dunn J, Grider MH. Physiology, Adenosine Triphosphate. [Updated 2022 Feb 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553175/
- National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 5957, Adenosine-5′-triphosphate. Retrieved April 2, 2022, from https://pubchem.ncbi.nlm.nih.gov/compound/Adenosine-5_-triphosphate.
- ATP – Energy Currency of the Cell – Structure and its Functions (byjus.com)
- Adenosine Triphosphate (ATP) – Definition, Structure and Function (biologydictionary.net)
- What is ATP – Biology Wise
- ATP Synthesis – NEET Biology Notes (byjus.com)
- ATP | Biology I (lumenlearning.com)
- Photophosphorylation: Definition and Types – QS Study
- Cyclic Photophosphorylation & Noncyclic Photophosphorylation (byjus.com)
- Photophosphorylation | BioNinja
- Photophosphorylation – an overview | ScienceDirect Topics
- Beta Oxidation – Definition, Steps and Quiz | Biology Dictionary
- Beta Oxidation of fatty acid: steps and examples – Online Biology Notes
- Why is fermentation less effective at making ATP? – Neeness
- Fermentation | General Biology at BCC (cuny.edu)
- What is ATP Hydrolysis? (with pictures) (thehealthboard.com)
- blobs.org – Hydrolysis of ATP
- Hydrolysis & Synthesis of ATP (1.6.2) | AQA A Level Biology Revision Notes 2017 | Save My Exams
- » How much energy is released in ATP hydrolysis? (bionumbers.org)
- ATP | Structure, Synthesis, Hydrolysis, Functions & Summary (alevelbiology.co.uk)
- Khakh, B. S., & Burnstock, G. (2009). The double life of ATP. Scientific American, 301(6), 84–92. https://doi.org/10.1038/scientificamerican1209-84
- Pelleg, A., Kutalek, S.P., Flammang, D. et al. ATPace™: injectable adenosine 5′-triphosphate. Purinergic Signalling 8, 57–60 (2012). https://doi.org/10.1007/s11302-011-9268-1
- Chu, X. Y., Wang, G., & Zhang, H. Y. (2021). ATP as an anti-aging agent: Beyond the energy reservoir. Drug discovery today, 26(12), 2783–2785. https://doi.org/10.1016/j.drudis.2021.09.022
- Hopfner, K.-P., Karcher, A., Shin, D. S., Craig, L., Arthur, L. M., Carney, J. P., & Tainer, J. A. (2000). Structural Biology of Rad50 ATPase: ATP-Driven Conformational Control in DNA Double-Strand Break Repair and the ABC-ATPase Superfamily. Cell, 101(7), 789–800. https://doi.org/10.1016/S0092-8674(00)80890-9
- Johannes Mehringer, Tuan-Minh Do, Didier Touraud, Max Hohenschutz, Ali Khoshsima, Dominik Horinek, Werner Kunz,Hofmeister versus Neuberg: is ATP really a biological hydrotrope?,Cell Reports Physical Science,Volume 2, Issue 2,2021,100343,ISSN 2666-3864, https://doi.org/10.1016/j.xcrp.2021.100343

I want the notes for MSc biochemistry 2nd semester