Complements are some special chemicals or protein components that are a part of our humoral immunity and aid in establishing antigen-antibody complexes, engulfing, degrading, and washing them away. They are found in the blood or attached near membranes. Eg. C1, C2, C3, C4 proteins, etc.
The complement system is a collection of several proteins which interact with each other to initiate a series of reactions to assist in the defense of our immune system.
Interesting Science Videos
What is Complement Fixation?
Definition- Complement fixation is one of the most important and one of the classical techniques for determining antigen-antibody complexes present in the testing sample.
In complement fixation, the antigen-antibody complex formed within the solution gets fixed with the complement proteins and the further process takes place, and hence it is named so.
Note: Only antigen alone or antibody alone cannot fix the complement. Fixing generally represents that the complement protein is in use.
It occurs in both conditions i.e. in vivo (in the human body) and in vitro (in artificial conditions like lab tests).
Complement Fixation Test Requirements
Samples such as serum or CSF (may or may not contain the specific antigens or antibodies of interest)
Known complementary antigens based on the component desired to be detected.
Complement Proteins: The native complement present in the sample is inactivated. Complement obtained from the serum of other organisms such as Guinea pig is added to the sample during the test.
Indicator System: Sheep erythrocytes or RBCs coated with antibodies (mainly derived from Rabbit serum) on the surface. These RBCs can also be called sensitized RBCs.
Complement Fixation Test Principle
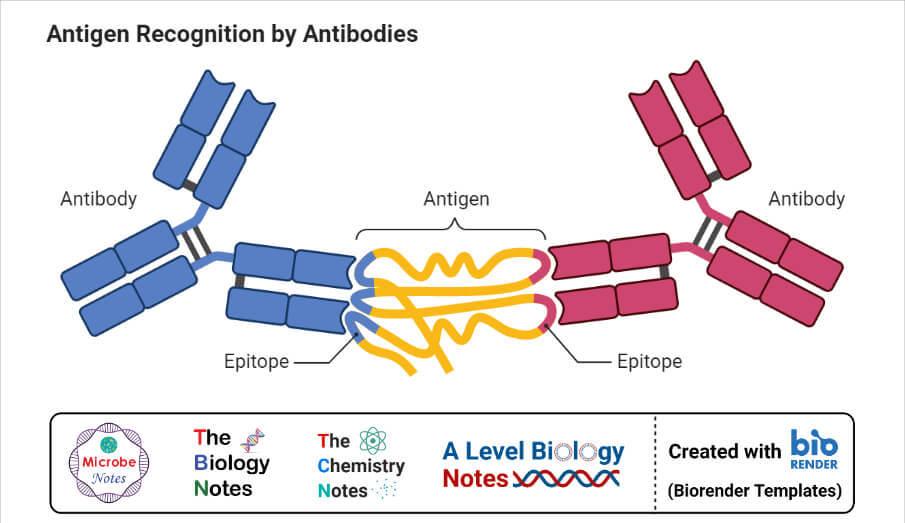
When antigen and antibody interact with each other, they form a complex called antigen-antibody (Ag-Ab ) complex. The complex then interacts with complement protein and gets fixed with it. After fixing, the complement degrades or gets cleaved into two fragments i.e. smaller and larger fragments.
For eg. C2 gets fragmented into C2a and C2b. The larger fragments or active sites remain attached to the Ag-Ab complex whereas smaller fragments separate and act as the signaling molecule. The signaling molecule provides the signal to macrophages for engulfment of complex and destruction of the antigen. It is a biological or in vivo mechanism.
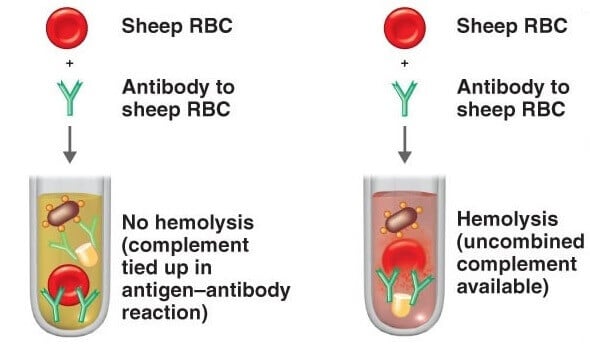
The complement fixation test is based on the principle that the Ag-Ab complex can only fix the complement and its effect on the hemolysis of RBC used in the indicator system.
If the sample contains desired antibodies or antigens, the Ag-Ab complex will be formed in the sample after the addition of a complementary reactant(antigen or antibody, based on the component being detected), and the indicator system will not be able to react to the added complement(as it already gets fixed with Ag-Ab complex) which results in no change in the indicator system.
No change in the indicator system refers to no lysis of RBC or no hemolysis.
Positive test
Antibody in sample + Antigen (added) + Complement → Ag-Ab Complex Fixed with Complement
Complement fixed Ag-Ab + Indicator System → No change (No hemolysis)
Negative test
Sample with no antibody + Antigen (added) + Complement → Free Complement
Antigen (added) + Antibody in indicator system (On RBC) → Ag-Ab complex
Ag-Ab complex + Complement → Fixed Complement System → Hemolysis
Complement Fixation Test Procedure
- A serum sample is taken.
- It is then heated at about 56 °C to remove the complement proteins already present in the sample.
- The serum is then adsorbed with washed sheep RBC. It prevents interference in the test by anti-RBC antibodies which are cross-reactive.
- Then the antigen and complement are added to the sample.
- It is then subjected to incubation at a temperature of 37 °C for 30 minutes. It provides conditions and time for the formation of the Ag-Ab complex.
- And the indicator system is then added and the sample is observed for change due to occurrence or non-occurrence of hemolysis.
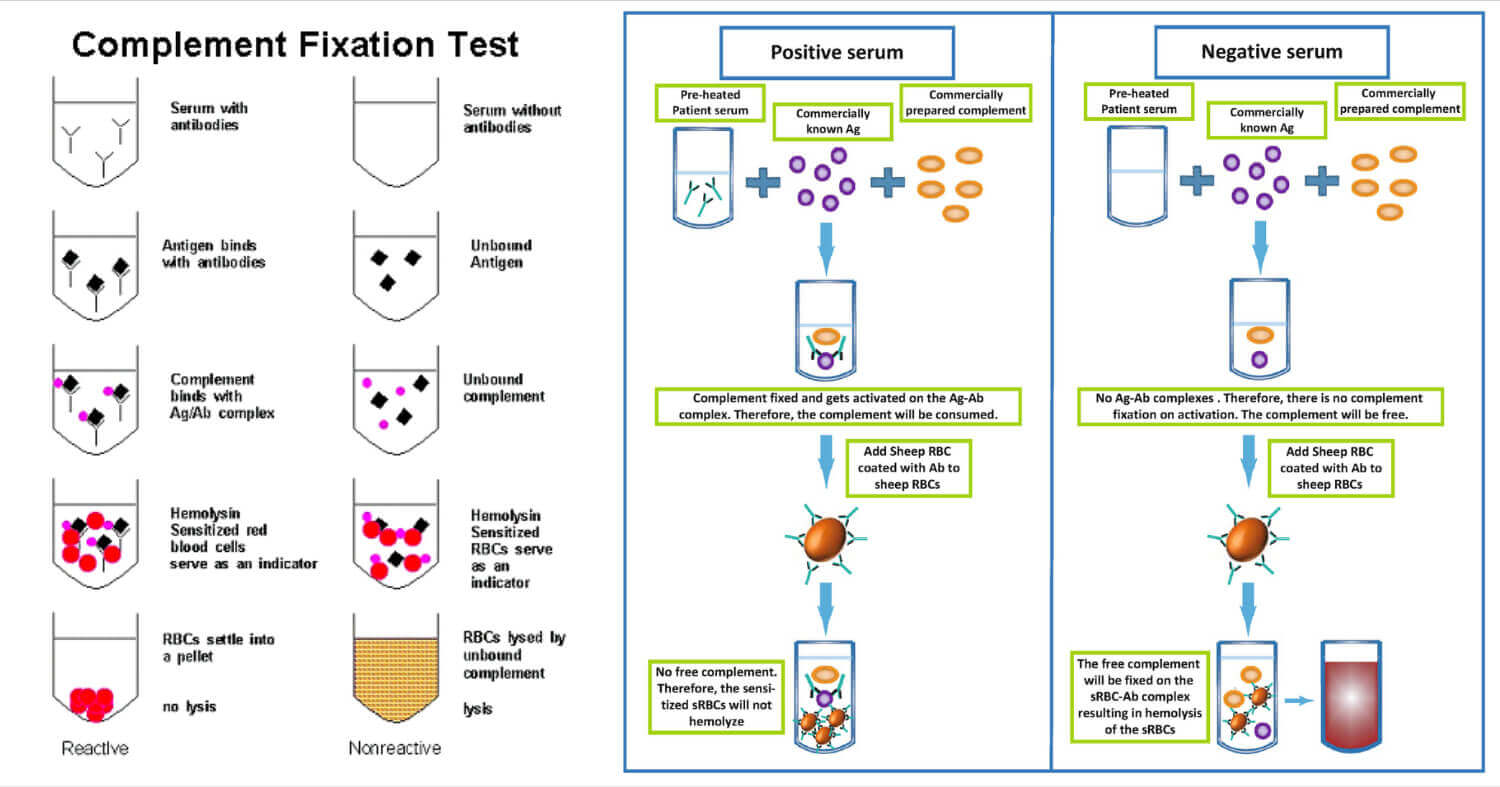
Complement Fixation Test Result Interpretation
If the sample contains the specific antibody or antigen of interest, there will be no change in the solution after the test and it will be considered a positive test. The non- hemolyzed sensitized RBC will remain as it is and settle down in the sample.
If there is some change in the appearance of the sample or solution during the test due to hemolysis, it will be considered a negative test.
Complement Fixation Test Applications
- Wasserman’s test is one of the complement fixation tests for the detection of syphilis. It is an antibody detection test.
- It can also be used for the detection of bacterial diseases caused by Mycobacterium pneumoniae, Bordetella pertussis, etc.
- It can be used for the detection of viral infections, and fungal infections such as Histoplasmosis, Cryptococcosis, etc.
Complement Fixation Test Advantages
- Interpretation of the result after the test is easier.
- It can be used for the detection of a very small number of antigen or antibody components in the sample.
- It can be used for the detection of a variety of infections.
- It has good sensitivity.
Complement Fixation Test Limitations
- It is one of the old methods not used much in current practices.
- It is slower and more complex in comparison to many easier rapid detection tests being used currently.
- It is difficult to perform and arrange the reagents used for it.
- Although it is one of the sensitive tests, it has less sensitivity than tests such as ELISA.
Types of Complement Fixation Test
Indirect complement fixation test
It is used when serums can’t fix guinea pig complement. These include avian sera (Parrot, Duck) and mammalian sera (Cat, Horse). Here, the test is set up in duplicate. After step 1, standard antiserum to an antigen that is known to fix complement is added to one set. If antibodies were not present in the test serum then the antigen would react with the standard antiserum fixing the complement. On the other hand, if antibodies are present in the test serum the antigen would be utilized in the first step. So, no reaction would occur between the standard antiserum and the antigen and therefore no fixation of complement would cause lysis of sheep red blood cells. Thus in this case hemolysis indicates a positive result.
Congulatinating complement absorption test
In this test, horse complement which is non-hemolytic is used. The indicator system used is sensitized sheep red blood cells mixed with bovine serum. The bovine serum contains a beta globulin called conglutinin would also combine with this complement causing agglutination (conglutination) of the sheep’s red blood cells, indicating a negative result. If horse complement is utilized by Ag-Ab reaction in the first step, agglutination of sensitized cells doesn’t occur.
Immune adherence
When some bacteria (such as Vibrio cholera or Treponema pallidum) combine with their specific antibody in the presence of complement and some particles such as erythrocytes or platelets, they adhere to the erythrocytes or platelets. This is called immune adherence. Immuno adherence facilitates phagocytosis of bacteria.
Immobilization test
Here antigen is incubated with the patient’s serum in presence of complement. If a specific antibody is present it would immobilize the antigen. Eg. Treponema palladium immobilization test, is considered the gold standard for the serodiagnosis of syphilis. A positive test shows serum to contain treponemal antibodies.
Cytolytic tests
The incubation of a live bacterium with its specific antibody in the presence of complement leads to the lysis of the bacteria cells. This is the basis of the Vibriocidal antibody test used to measure anti-cholera antibodies.
References
- Goldsby R.A., Kindt T.J., Osborne B.A., (1999) Kuby Immunology, 4th edition, W.H.Freeman & Co Ltd.
- Parija S.C., (2009), Textbook of Microbiology and Immunology, 2nd edition, Elsevier, a division of Reed Elsevier India Private Limited
- Jeffrey K., Complement Fixation Test Introductory Immunology (Second Edition), 2019Miller, V. B. (1930). Tests for Syphilis: An Explanation of the Wasserman Test. The American Journal of Nursing, 30(6), 707–712. https://doi.org/10.2307/3410619.

thanks for contributing