Interesting Science Videos
Habitat of Chlamydia trachomatis
- It is an obligate intracellular human pathogens.
- Humans are the only natural host.
- It cannot survive outside of a eukaryotic host.
- Chlamydia trachomatis is transmitted by oral, vaginal or anal sex, and can also be transmitted from mother to newborn during a vaginal delivery.
- They can cause discharge from the penis, pain and burning during urination, infection or inflammation in the ducts of testicles, and tenderness or pain in the testicles.
Morphology of Chlamydia trachomatis
- It is a weak Gram-negative bacteria.
- It also contains LPS, which helps cause damage to the host’s body.
- It lacks a peptidoglycan cell wall.
- It lacks muramic acid that is found in the cell walls of most other bacteria.
- It is non-sporing.
- They are non-motile.
- It has a coccoid or rod shape.
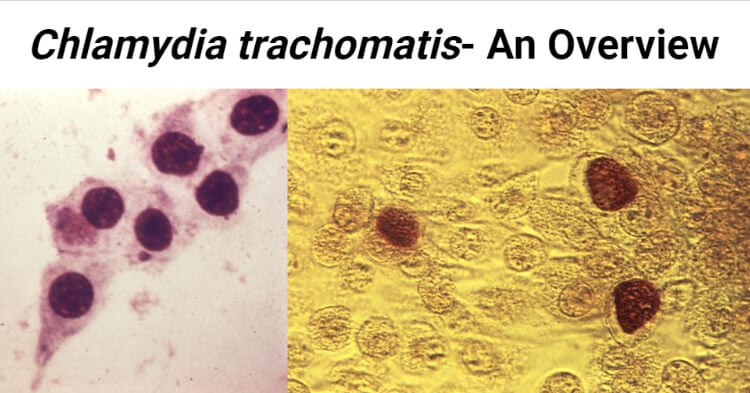
- They exist in two morphological forms: small infectious elementary bodies 300 nm – 400 nm in diameter and larger replicating reticulate bodies 800 nm – 900 nm in size.
- The elementary body is the dispersal form, which is analogous to a spore.
- The dispersal form is about 0.3 um in diameter.
- The reticulate body divides through binary fission at approximately 2-3 hours per generation.
- The reticulate body has an incubation period of 7-21 days in the host.
- The reticulate body contains no cell wall and is detected as an inclusion in the cell.
Genome of Chlamydia trachomatis
- It have an extra-chromosomal plasmid.
- It has a genome that consists of 1,042,519 nucleotide base pairs.
- It has approximately 894 likely protein coding sequences.
- All the isolates are about 7,500 nucleotides long.
- It has eight open reading frames computer-predicted to code for proteins of more than 100 amino acids.
Cultural Characteristics of Chlamydia trachomatis
- Chlamydia trachomatis is an obligate intracellular bacterium that grows within a mammalian host cell for survival.
- The bacteria cannot be cultured via conventional cultural methods on bacteriological medium. This also makes C. trachomatis a difficult organism to grow and maintain in most laboratories.
- Until 1965, inoculation in the yolk sac of the embryonated eggs was the only method for the isolation and propagation of the organism.
- Nowadays, however, the tissue culture system has been developed that allows easier laboratory culture and maintenance of most Chlamydia species.
- The tissue system can be used for the isolation, culture and purify large quantities of Chlamydia species from clinical and laboratory stock cultures.
- Laboratory works related to C. trachomatis are to be performed by following appropriate safeguards as aerosols might be produced during centrifugation and sonication posing a great risk of laboratory infections.
- C. trachomatis can be isolated from various clinical samples like urethral, urogenital and rectal swabs.
- McCoy monolayer cells are infected with C. trachomatis to prepare frozen stocks of amplified clinical isolates.
- The incubation of the chlamydial cultures occurs within the optimal temperature of 35°C to 37°C.
- The cells of Chlamydia reproduce by binary fission in the cytoplasmic vacuole surrounded by the cytoplasmic membrane.
Click here for Biochemical Test of Chlamydia trachomatis
Pathogenesis of Chlamydia trachomatis
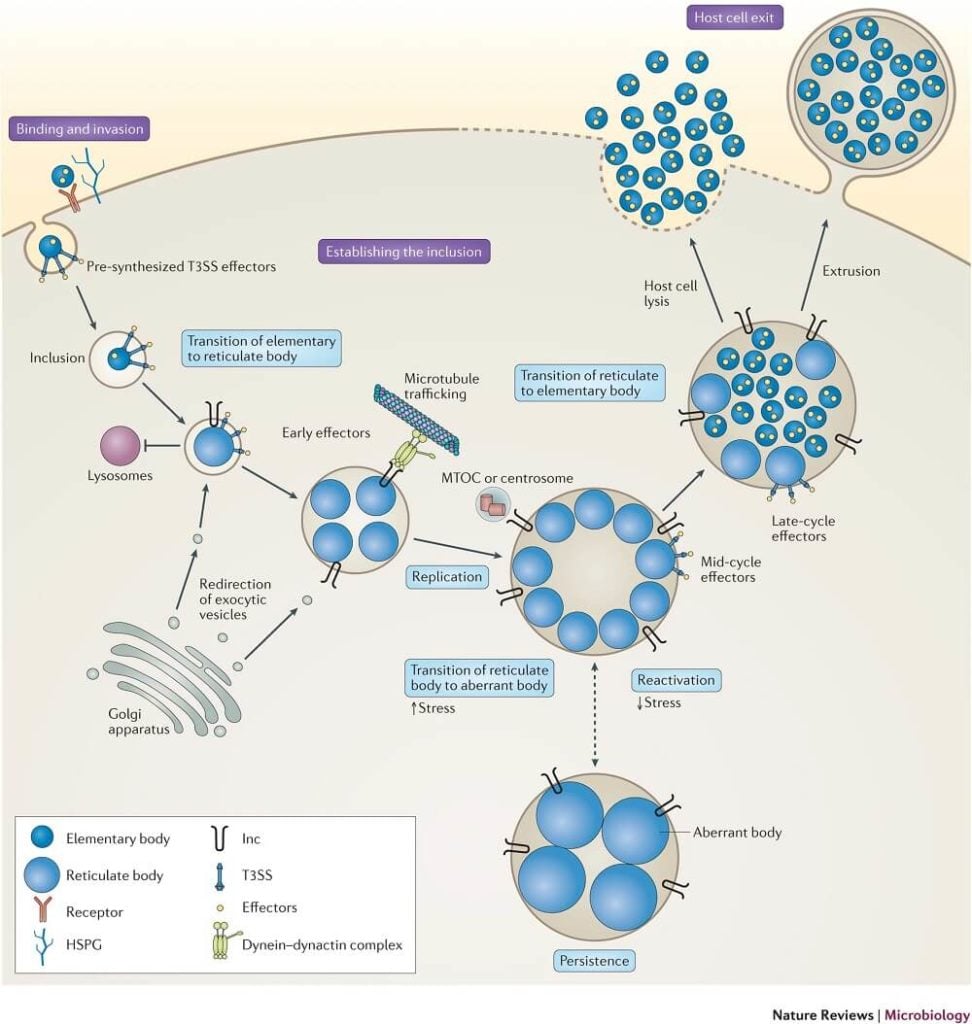
- Chlamydia are acquired by direct contact with mucous membranes or abraded skin, that is, by sexual contact or by direct inoculation into the eye in the case of trachoma or neonatal conjunctivitis.
- Two forms of the organism are needed for infection and disease to occur: the infectious, extracellular form called an elementary body (EB) and the noninfectious but metabolically active intracellular form called a reticulate body (RB).
- Receptors for EBs are primarily restricted to nonciliated columnar, cuboidal, and transitional epithelial cells, which are found on the mucous membranes of the urethra, endocervix, endometrium, fallopian tubes, anorectum, respiratory tract, and conjunctivae.
- Infection is initiated by attachment of EBs to the apical surfaces of epithelial cells of the conjunctiva, respiratory, gastrointestinal, or urogenital tracts, followed by entry by receptor-mediated endocytosis.
- The EBs quickly modify their early endosomal membrane to exit the endosomal pathway, thereby avoiding fusion with lysosomes and traffic on microtubules to the peri-Golgi/ nuclear hof region.
- The EB-containing endosomes of C. trachomatis then fuse homotypically with one another to form their one nascent microcolony called an inclusion.
- The EBs then transform into RBs, the chromosome becomes relaxed and transcriptionally active, and metabolic growth and binary fission ensue to generate progeny.
- RBs obtain their nutrients from the epithelial cytoplasm using at least two strategies: (1) insertion of chlamydial proteins (Incs) into the inclusion membrane, some of which likely have autotransporter functions and (2) insertion of a unique appendage through the inclusion membrane.
- RBs are osmotically fragile and do not survive outside their inclusion nor can they bind to epithelial cells. Thus, to perpetuate the infectious process, RBs must mature back into infectious EBs before escaping from infected cells.
- The inclusion membrane may then fuse with the host plasma membrane to release chlamydiae, or the host cell, depleted of nutrients and energy, may lyse.
- Luminal C. trachomatis progeny are released at the apical surfaces of polarized columnar epithelial cells to spread canalicularly to the upper genital tract, whereas the invasive LGV serovars are released at the basal domain into the submucosa enroute to the regional lymph nodes.
- Infection of epithelial mucosal cells with C. trachomatis has been shown to generate several cytokines, including interleukin-1α (IL-1α), IL-6, IL-8, GRO-α and granulocyte–macrophage colony stimulating factor (GM–CSF), which generate and sustain an inflammatory response.
- Inflammatory mediators and chemokines produced in infected epithelial cells serve as initial triggers for an influx of leukocytes including neutrophils, natural killer cells, dendritic cells, monocytes, and lymphocytes.
- Infected epithelial cells and early infiltrating natural killer cells activate antigen presenting cells into programming the cell-mediated immune response.
- As the host immune response develops, active sites of infection show an infiltration of lymphocytes, plasma cells, and macrophages.
- IFNs, in particular IFN-α, play an important part in the immune response to chlamydial infection by inhibiting intracellular replication at the RB stage through tryptophan depletion.
- However, this may result in continuing secretions of chlamydial antigens, leading to further sensitization and also induction of persistent non-replicating infection.
- Resistance to infection and clearance of primary infection are due in large part to T-cell function, with both CD4 and CD8 cells having a protective role.
- MOMP-specific T-helper 1 (TH1) CD4 cells may have an important role in immunity, while Hsp60-specific TH2 CD4 cells are associated with the pathological sequelae of persistent infection.
- A chlamydial heat-shock protein (hsp 60), elicits antibody responses that are associated with the damaging sequelae of C. trachomatis infections in both the eye and genital tract.
- A period of chronic inflammation ensues, with the development of sub-epithelial follicles, and this leads eventually, in some cases, to fibrosis and scarring.
- Follicles contain typical germinal centres, consisting predominantly of B lymphocytes, with T cells, mostly CD8+, in the parafollicular region.
- The inflammatory infiltrate between follicles comprises plasma cells, dendritic cells, macrophages, and polymorphonuclear leucocytes, with T and B lymphocytes .
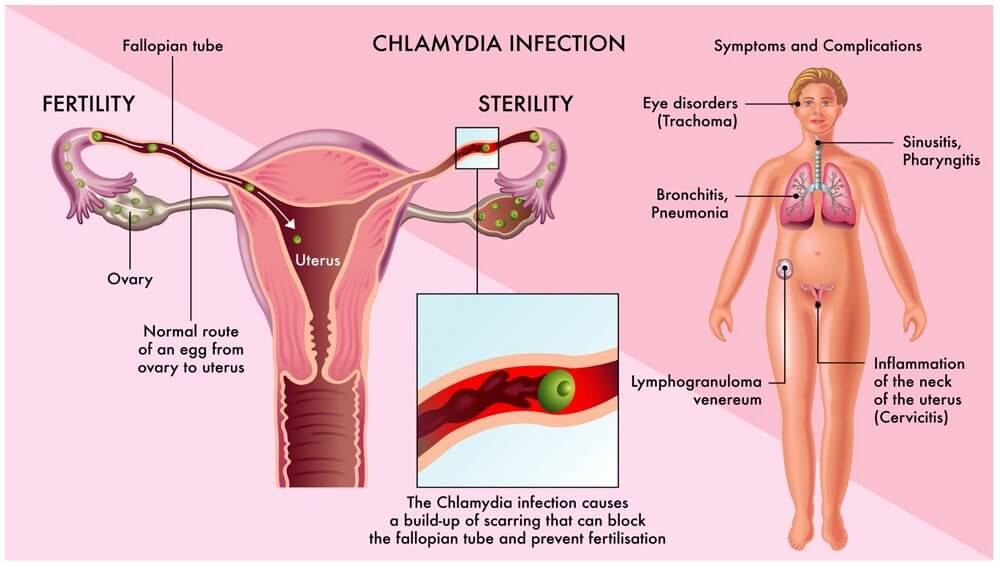
- The scarring process is responsible for much of the morbidity associated with C. trachomatis, in both the genital tract and the eye.
- Studies of gene expression at the site of ocular infection have shown the importance of innate immune pathways and NK (natural killer) cell activation, and suggest that matrix metalloproteinases 7 and 9 play an important role in the scarring process.
- It is particularly likely to be seen after repeated infections.
- Polymorphisms in immune response genes encoding tumour necrosis factor-α, interferon-γ and interleukin-10 are associated with the development of severe scarring following ocular C. trachomatis infection.
- Fibrosis is seen at a late stage, typically in trachoma and pelvic inflammatory disease.
Clinical Manifestations of Chlamydia trachomatis
A. Trachoma
- It is a chronic keratoconjunctivitis that begins with acute inflammatory changes in the conjunctiva and cornea and progresses to scarring and blindness.
- The C. trachomatis serovars A, B, Ba, and C are associated with clinical trachoma.
- Trachoma is transmitted through direct contact (fingers and fomites) with discharges from the eyes of the infected patients or indirect contact through contaminated clothes or flies.
- The incubation period for chlamydial conjunctival infection is 3–10 days.
- The earliest symptoms of trachoma are lacrimation, mucopurulent discharge, conjunctival hyperemia, and follicular hypertrophy.
- Acute infection presents as a follicular conjunctivitis, with congestion and oedema affecting both the palpebral and bulbar conjunctivae.
- There is papillary hyperplasia, giving the palpebral conjunctiva a velvety appearance.
- In hyperendemic areas, infection tends to be more pronounced in the upper lid.
- Follicles rupture to leave shallow pits termed Herbert’s pits.
- Keratitis develops in the cornea.
- Recurrent infection leads to conjunctival scarring or cicatrization which may occur at sclera-conjunctiva junction (limbal scarring) or on palpebral conjunctiva and new vessel formation (pannus).
- Palpebral conjunctival scarring (cicatrisation) leads to in-turning of the eyelashes (entropion), which scrape the bulbar corneal surface (trichiasis).
- It is the cycle of recurrent infection, with conjunctival scarring and pannus extending over the cornea, which results in impaired vision or blindness.
B. Genital infections
- C. trachomatis serovars D–K cause sexually transmitted diseases, and may also produce infection of the eye (inclusion conjunctivitis).
Infection in men
- Genital infection in men presents most commonly as non-gonococcal urethritis (NGU).
- C. trachomatis is detectable in the urethra of up to 50% of men with symptomatic non-gonococcal urethritis.
- The incubation period is 7–21 days, compared to 2–5 days for gonorrhoea.
- Patients present with a history of dysuria, usually accompanied by a mild to moderate mucopurulent urethral discharge, acute epididymitis.
- Patients present with unilateral scrotal pain, swelling and tenderness, often accompanied by fever.
- Proctitis and proctocolitis may occur in men and women, although these infections appear to be most common in men who have sex with men.
Infection in women
- C. trachomatis typically infects the columnar epithelial cells of the endocervix.
- Infection is associated with a mucopurulent discharge from the cervix visible on speculum examination, and with hypertrophic cervical ectopy that tends to bleed on contact.
- C. trachomatis has been implicated as a cause of the urethral syndrome, characterized by dysuria, frequency and sterile pyuria.
- The clinical manifestations include bartholinitis, cervicitis, endometritis, perihepatitis, salpingitis, and urethritis.
- Spread to the peritoneum may result in perihepatitis (the Curtis–Fitz-Hugh syndrome), which may be confused with acute cholecystitis in young women.
- Many women, if untreated, will go on to develop serious long-term sequelae of infection such as PID, infertility and ectopic pregnancy.
C. Neonatal inclusion conjunctivitis (ophthalmia neonatrum)
- The incubation period for chlamydial infection is significantly longer (6–21 days).
- Chlamydial ophthalmia presents with a watery ocular discharge, which may progress to a purulent conjunctivitis with marked periorbital oedema.
- Because the conjunctiva at birth lacks a lymphoid layer, follicles do not develop initially but may be seen after 3–6 weeks.
- Unlike trachoma, the lower conjunctival surface is more heavily infected than the upper.
- Swelling of the infant’s eyelid, hyperemia, and purulent discharge characterize the condition
- Conjunctival scarring and corneal vascularization occurs in untreated infections of long duration
D. Infant pneumonia
- Infant pneumonia caused by C. trachomatis is seen in infants between 4 and 16 weeks of age.
- The incubation period is variable but usually takes 2–3 weeks after birth.
- Pneumonitis develops when organisms present in the conjunctiva pass down the nasolacrimal duct into the pharynx.
- The condition is characterized by respiratory symptoms, such as rhinitis with cough and wheezing
- Infection via the Eustachian tube may cause otitis media.
E. Adult inclusion conjunctivitis
- Adult inclusion conjunctivitis results from the infection with C. trachomatis strains associated with genital infection (A, B, Ba, and D–K).
- This infection is more frequently seen in sexually active adults.
- The condition can also occur in neonates.
- A uniocular and less commonly binocular red eye, ocular discharge, marked hyperemia, papillary hypertrophy, and a predominant follicular conjunctivitis are the important manifestations.
- The condition if untreated progresses to a chronic remittent course, keratitis, and possible iritis.
F. Reactive arthritis
- Arthritis occurring with or soon after non-gonococcal urethritis is termed ‘sexually acquired reactive arthritis’.
- It is believed that Reiter syndrome (urethritis, conjunctivitis, polyarthritis, and mucocutaneous lesions) is initiated by genital infection with C. trachomatis.
G. Lymphogranuloma venereum (LGV)
- C. trachomatis serovar Ll, L2, L2a, L2b and L3 are the agents of lymphogranuloma venereum (LGV).
- The clinical course of LGV can be divided into three stages:
- Primary stage
- Painless papule, ulcer or vesicle develops on the penis or vulva after an incubation period of 3 days to 6 weeks.
- The primary lesion is self-limiting and may pass unnoticed by the patient
- Secondary stage
- This occurs some weeks after the primary lesion.
- It may involve the inguinal lymph nodes, or the anus and rectum.
- LGV proctitis occurs in those who practice receptive anal intercourse, probably due to direct inoculation.
- The cardinal feature of the inguinal form of LGV is painful, usually unilateral, inguinal and/or femoral lymphadenopathy (bubo).
- Enlarged lymph nodes are usually firm and often accompanied by fever, chills, arthralgia and headache.
- Small discrete areas of necrosis surrounded by proliferating epithelioid and endothelial cells, which may enlarge to form stellate abscesses that may coalesce and break down to form discharging sinuses.
- In women, signs include a hypertrophic suppurative cervicitis, backache and adnexal tenderness.
- Clinical features of anorectal disease include a purulent anal discharge, pain and bleeding due to an acute haemorrhagic proctitis or proctocolitis, often with fever, chills and weight loss.
- Enlarged inguinal nodes may also be palpable.
- Third stage
- Occurs in untreated cases, especially in women and homosexual men.
- Chronic untreated LGV leads to fibrosis, which may cause lymphatic obstruction and elephantiasis of the genitalia in either sex due to impaired lymphatic drainage or rectal strictures, rectosigmoid obstruction and fistula formation
- Esthiomene-the vulva, scrotum or penis may undergo edematous granulomatous hypertrophy.
Laboratory Diagnosis of Chlamydia trachomatis
Specimen
- Urethral discharge, cervix swab, rectum, oropharynx, and conjunctiva swab are the frequently collected specimens.
- In addition, other specimens such as, blood, urine, respiratory secretions, sputum, lung, and other tissues are collected and examined.
- Pus from bubo is also useful for diagnosis of LGV.
Microscopy
- Demonstration of chlamydial inclusion bodies stained by Giemsa, Castaneda, Machiavello, Gimenez stains or Lugol’s iodine.
- C. trachomatis infections of conjunctiva, urethra, and cervix are diagnosed by demonstration of typical reniform inclusion bodies surrounding the nucleus.
- Direct Immunofluorescence test (DIF) is used as for direct detection of inclusion bodies in clinical material, particularly from the genital tract and eye.
- In DIF, fluorescent tagged monoclonal antibodies directed against group-specific LPS antigen or species-specific MOMP antigens are added.
Culture
- Isolation of C. trachomatis in cell cultures is the more specific method for diagnosis of C. trachomatis infection.
- Centrifugation of specimens onto cycloheximide treated McCoy or HeLa cell monolayers, followed by incubation and then staining with a fluorescent monoclonal antibody or with a vital dye, to detect inclusions, has been widely used for the diagnosis of C. trachomatis infection.
Antigen detection
- Two general approaches have been used to detect chlamydial antigens in clinical specimens: direct immunofluorescence staining with fluorescein-conjugated monoclonal antibodies and enzyme-linked immunosorbent assays.
- In both assays, antibodies are used that have been prepared against either the chlamydial MOMP or the cell wall LPS.
- The DFA uses monoclonal antibodies directed against a species-specific antigen on the chlamydial MOMP.
- The EIA detects the presence of genus-specific antigens extracted from EBs in the specimen.
Nucleic Acid-Based Tests
- Nucleic acid probe tests most commonly measure the presence of a species-specific sequence of 16S ribosomal RNA.
- The advantage of these tests is that the nucleic acid does not have to be amplified, making the tests rapid and relatively inexpensive; however, these tests are relatively insensitive for the detection of small numbers of chlamydiae.
- Various methods available are:
- Polymerase chain reaction (PCR)
- Ligase chain reaction (LCR)
- Transcription-mediated amplification (TMA)
- Strand displacement assay (SDA).
Antibody Detection
- Serologic testing is of limited value in the diagnosis of C. trachomatis urogenital infections in adults because the test cannot differentiate between current and past infections.
- C. trachomatis IgM antibody is the ‘gold standard’ for the diagnosis of chlamydial pneumonia in babies.
- Antibody tests for the diagnosis of LGV can be helpful.
- Infected patients produce a vigorous antibody response that can be detected by complement fixation (CF), microimmunofluorescence (MIF), or enzyme immunoassay (EIA).
Treatment of Chlamydia trachomatis Infection
- Tetracyclines and macrolides are the mainstay of treatment.
- Tetracyclines (eg, doxycycline) are commonly used in nongonococcal urethritis and in nonpregnant infected women.
- Azithromycin is effective and can be given to pregnant women.
- Ophthalmia neonatorum and neonatal pneumonia due to C. trachomatis should be treated with erythromycin.
- Erythromycin may be administered orally and topically for treatment of ophthalmia neonatorum.
- Systemic erythromycin is effective treatment in severe cases.
- Recommended treatment for LGV is doxycycline or erythromycin.
- Azithromycin has been used successfully in some cases.
- Ocular infection can be effectively treated with a single oral dose of azithromycin.
Prevention and Control of Chlamydia trachomatis Infection
- Health education and condom promotion, especially for the youngest sexually active age groups may help to reduce the incidence of genital C. trachomatis infection.
- Periodic screening of high risk groups, such as young women having multiple sex partners.
- Use of barrier methods of contraception such as condoms.
- Chlamydia conjunctivitis and genital infections are prevented through the use of safe sex practices and the prompt treatment of symptomatic patients and their sexual partners.
- The blindness associated with advanced stages of trachoma can be prevented only by prompt treatment of early disease and the prevention of re-exposure.
- Screening of mother giving birth to a child for Chlamydial infections.
References
- Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201 Suppl 2(Suppl 2):S114-S125. doi:10.1086/652397
- Brunham, R., Rey-Ladino, J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol 5, 149–161 (2005). https://doi.org/10.1038/nri1551
- Elwell, C., Mirrashidi, K. & Engel, J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol 14, 385–400 (2016). https://doi.org/10.1038/nrmicro.2016.30
- Mohseni M, Sung S, Takov V. Chlamydia. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537286/
- O’Connell CM, Ferone ME. Chlamydia trachomatis Genital Infections. Microb Cell. 2016;3(9):390-403. Published 2016 Sep 5. doi:10.15698/mic2016.09.525
- Witkin SS, Minis E, Athanasiou A, Leizer J, Linhares IM. Chlamydia trachomatis: the Persistent Pathogen. Clin Vaccine Immunol. 2017;24(10):e00203-17. Published 2017 Oct 5. doi:10.1128/CVI.00203-17
- Malhotra M, Sood S, Mukherjee A, Muralidhar S, Bala M. Genital Chlamydia trachomatis: an update. Indian J Med Res. 2013;138(3):303-316.
- Smolarczyk K, Mlynarczyk-Bonikowska B, Rudnicka E, et al. The Impact of Selected Bacterial Sexually Transmitted Diseases on Pregnancy and Female Fertility. Int J Mol Sci. 2021;22(4):2170. Published 2021 Feb 22. doi:10.3390/ijms22042170.

whoah this blog is excellent i love reading your posts. Keep up the great work! You know, lots of people are looking around for this info, you can help them greatly.
Hi Mauricio,
Thank you so much for liking our website. We also hope this website and posts will be useful to a maximum number of people.