In 1984, Bolton et al. stated charcoal might efficiently substitute blood in a culture medium for isolating Campylobacter spp. Endtz et al. later confirmed there is a higher isolation rate for Campylobacter while using the charcoal selective medium.
Interesting Science Videos
Composition of Charcoal Selective Medium
| Ingredients | Gm/Liter |
| Beef extract | 10.0g |
| Gelatin Peptone | 10.0g |
| Sodium Chloride | 5.0g |
| Casein Peptone | 3.0g |
| Charcoal | 4.0g |
| Sodium desoxycholate | 1.0g |
| Ferrous sulfate | 0.25g |
| Sodium Pyruvate | 0.25g |
| Cycloheximide | 100.0mg |
| Cefoperazone | 32.0mg |
| Hematin | 32.0mg |
| Vancomycin | 20.0mg |
| Agar | 12.0g |
pH 7.4± 0.2 (at 25°C)
Principle of Charcoal Selective Medium
The medium consists of beef extract which supplies nitrogen, carbohydrates, vitamins, and other nutrients required for the growth of Campylobacter. Gelatin and casein peptones provide nutrients in the form of amino acids and peptides. The charcoal, hematin, sodium pyruvate, and ferrous sulfate improve the aerotolerance of Campylobacter species; it has been suggested that these supplements act as quenching agents of photochemically-produced toxic oxygen derivatives. Charcoal is a detoxifying agent and reduces oxygen tension. Sodium chloride provides essential electrolytes maintaining osmotic equilibrium thereby maintaining the integrity of cells. Sodium desoxycholate is a selective medium that inhibits some bacteria. Cefoperazone is a cephalosporin antibiotic that suppresses the growth of gram-negative enteric bacilli and some gram-positive species. Vancomycin is a glycopeptide antibiotic that inhibits many species of gram-positive bacteria. Cycloheximide is an antifungal agent that inhibits yeast and mold.
Preparation of Charcoal Selective Medium
- Add components to distilled/deionized water and bring volume to 1000 ml.
- Mix thoroughly. Gently heat and bring to boiling with frequent stirring.
- Autoclave for 15 min at 15 psi pressure at 121°C.
- Cool to 45-50°C.
- Pour into sterile Petri dishes or distribute into tubes.
- Shake flask while dispensing to keep charcoal in suspension.
Result Interpretation on Charcoal Selective Medium
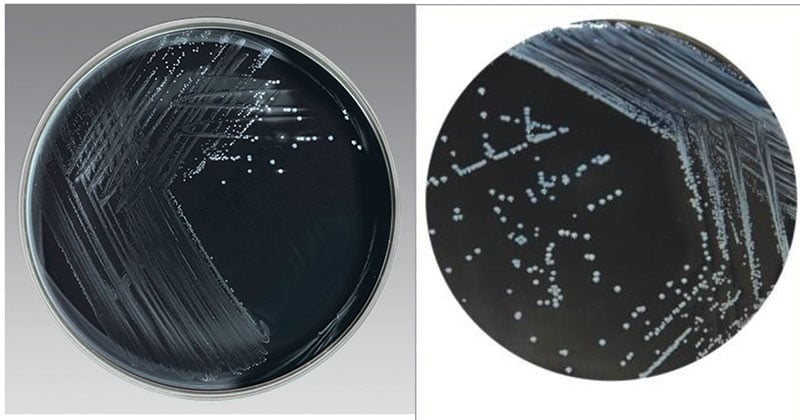
Image Source: Thermo Scientific and HIMEDIA.
- Campylobacter jejuni produces two types of colonies.
- One is small, raised, grayish-brown, smooth and glistening with an entire translucent edge.
- The other colony type is flat, mucoid, translucent, grayish and has an irregular edge.
- Flat, irregular, or spreading colonies are observed on fresh medium.
- Some strains appear as the thin film on the agar or form colonies that tail along the line of the streaking.
- On less fresh medium, colonies are 1-2 mm in diameter, round; convex and glistening colonies are formed. Colonies can be yellowish to grey or pinkish in color and are non-hemolytic.
Uses of Charcoal Selective Medium
- It is used for selective isolation and presumptive identification of Campylobacter species from food and human fecal specimens.
Limitations of Charcoal Selective Medium
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- Extending incubation to 72 hours may increase the isolation rate of the Campylobacter
- Campylobacter coli, Campylobacter fetus and some strains of the Campylobacter jejuni are inhibited by the cephalosporins.
- Incubation of Campylobacter jejuni should be carried out at 42°C as it is a thermophilic organism. Higher temperature imparts selectivity by inhibiting accompanying microflora and promotes the growth of Campylobacter jejuni.
- The agents in a selective medium may inhibit some strains of the desired species or permit growth of a species it was designed to inhibit, especially if the species is present in large numbers in the specimens. Specimens cultured on selective media should, therefore, should also be cultured on nonselective media to obtain additional information and help ensure recovery of potential pathogens.
References
- Dalynn Biologicals
- Hardy Diagnostic
- Thermo fisher
- Himedia
- Becton, Dickinson and Company
- http://legacy.bd.com/ds/technicalCenter/inserts/8813781(02)(201202).pdf
- Ronald M. Atlas and James W. Snyder (2014). Handbook of media for clinical and public health microbiology. CRC Press. Taylor & Francis Group, LLC.
- Karmali MA, Simor AE, Roscoe M, Fleming PC, Smith SS, Lane J (1986). Evaluation of a blood-free, charcoal-based, selective medium for the isolation of Campylobacter organisms from feces. Journal of Clinical Microbiology 23(3):456-459.
