Burkholderia pseudomallei is a Gram-negative, motile, non-spore-forming, aerobic Betaproteobacteria of the Pseudomonadota phylum in the Burkholderiaceae family.
It is an environmental saprophytic bacteria naturally found in soil and water. However, B. pseudomallei have shifted from soil bacteria to deadly intracellular pathogenic bacteria causing ‘melioidosis’ in humans which has a mortality rate of 20 to 40% even with an early diagnosis and appropriate therapy.
It can survive in harsh environmental conditions like extreme temperature, extreme pH, dry soils, and nutrient deficient conditions like distilled water for several days and cause deadly disease, so it has been listed as a ‘Tier 1 select agent’ and potential bioweapon by the US CDC (Center for Disease Control and Prevention).
| Domain | Bacteria |
| Phylum | Pseudomonadota |
| Class | Betaproteobacteria |
| Order | Burkholderiales |
| Family | Burkholdericeae |
| Genus | Burkholderia |
| Species | B. pseudomallei |
Interesting Science Videos
Habitat of Burkholderia pseudomallei
- Burkholderia pseudomallei are soil bacteria and they are commonly found in moist soils of tropical and sub-tropical regions. They are reported from rice paddy fields, marshy areas, and wetlands, soils around water sources, and even from stagnant waters and waters of rivers.
- B. pseudomallei can infect humans and animals like monkeys, pigs, goats, and sheep and survive within their bodies.
Morphology of Burkholderia pseudomallei
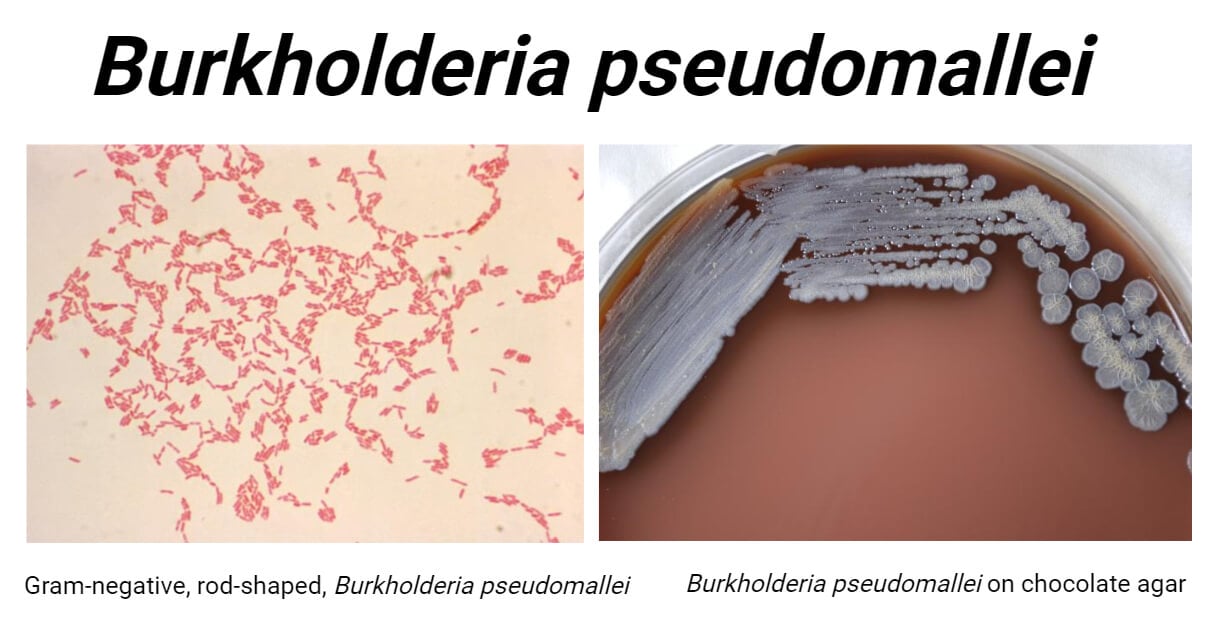
- It is a Gram-negative rod-shaped bacterium measuring about 2 – 5 μm in length and about 0.4 – 0.8 μm in width.
- It shows bipolar staining and gives its characteristic ‘safety pin’ appearance under a microscope.
Cultural Media for Burkholderia pseudomallei
- B. pseudomallei is a non-fastidious bacterium and can grow in diverse culture mediums used for other Gram-negative bacteria like MacConkey Agar, Blood Agar, Trypticase Soy Agar, Eosin Methylene Blue (EMB) Agar, Nutrient Agar, etc. However, B. pseudomallei is easily overgrown by other commensals or contaminants so, it is always necessary to use a selective medium for the isolation of Burkholderia pseudomallei.
- Ashdown’s medium, Burkholderia cepacia medium, and Burkholderia pseudomallei selective agar (BPSA) are the three available selective mediums that are routinely used in microbiology laboratories. Among these three media, Ashdown’s medium is considered superior for its selectivity of B. pseudomallei strains. However, quick growth (after 18 to 24 hours) can be seen in Burkholderia cepacia medium and BPSA.
Cultural Characteristics of Burkholderia pseudomallei
- In Ashdown’s medium B. pseudomallei gives large, wrinkled, circular, wet/mucoid (if mucoid strains) and dry (if non-mucoid strains), flat, purple-colored colonies after 48 hours of incubation at 37 to 40°C in air. These wrinkled purple colonies give presumptive identification of B. pseudomallei.
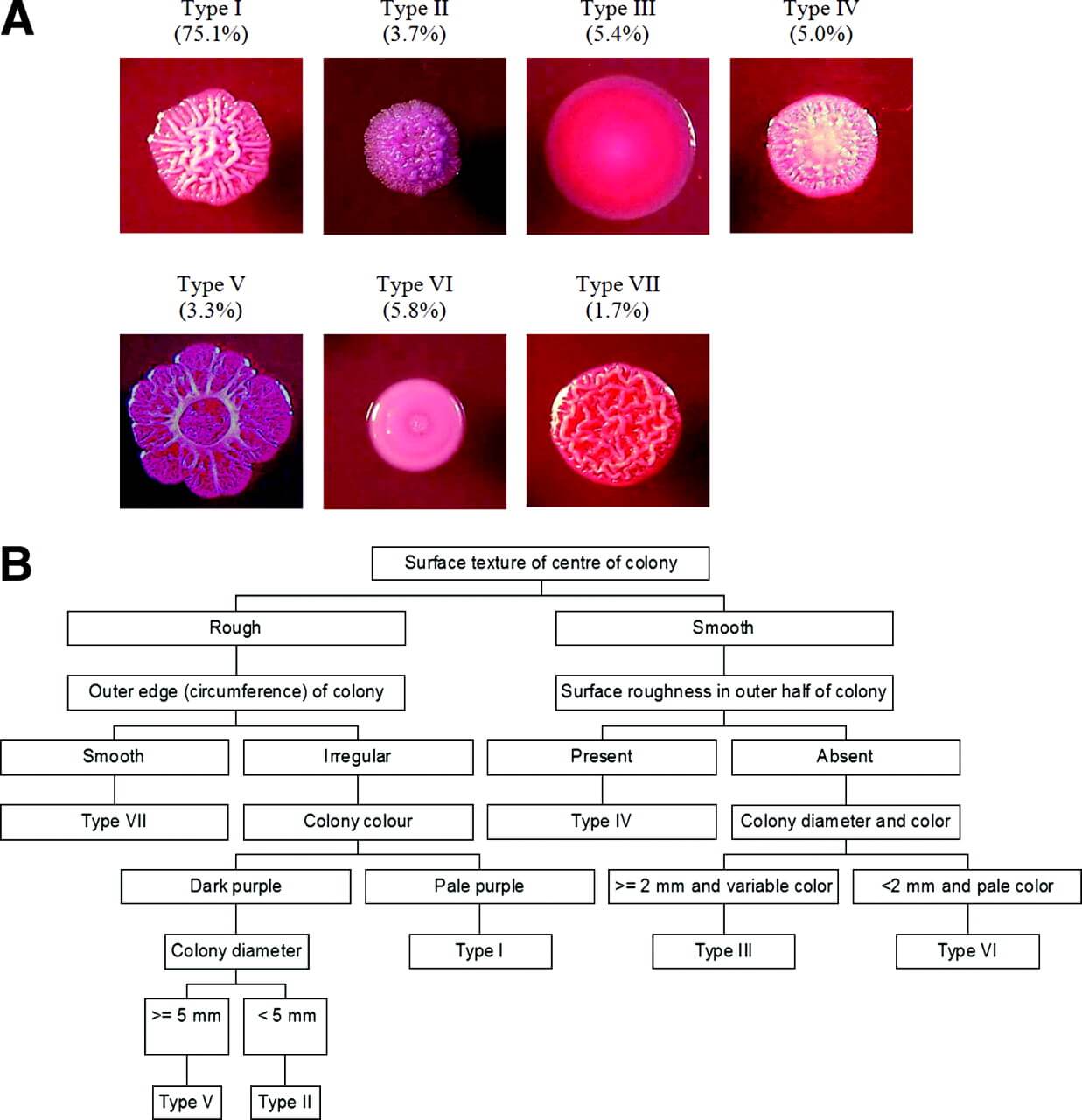
- In BPSA, similar colony morphology is seen in large, wrinkled, circular, mucoid or dry, flat, mauve/purple colonies by 48 hours and become deep purple on further incubation in the air at around 37°C.
- In MacConkey agar, large, smooth, pink, moist/dry colonies are seen after overnight incubation at 37°C, and these colonies will become wrinkled after 2 days of incubation.
- In Blood Agar, the colonies will be large, smooth, creamy, moist/dry colonies after 24 hours of aerobic incubation at around 37°C and the colonies will eventually be wrinkled after 48 hours.
Biochemical Characteristics of Burkholderia pseudomallei
General Biochemical Tests
| Characteristics | Burkholderia pseudomallei |
| Capsule | Mostly Positive (Few are unencapsulated) |
| Catalase | Positive (+) |
| Citrate (Simmons) | Variable {most show negative when incubated at ≥ 30°C} |
| Flagella | Positive (+) |
| Gas | Negative (-) |
| Gram Staining | Gram Negative Bacilli Bipolar Staining Safety pin appearance |
| H2S (Hydrogen Sulfide) | Negative (-) |
| Indole | Negative (-) |
| Motility | Motile |
| Nitrate Reduction | Positive (+) |
| Oxidase | Positive (+) |
| OF (Oxidative Fermentation) | Aerobic |
| Spore | Negative (-) |
| Triple Sugar Iron (TSI) | K/K (Alkaline/Alkaline) (Red/Red) |
| Urease | Negative (-) |
Carbohydrate Fermentation Tests
| Characteristics | Burkholderia pseudomallei |
| Arabinose | Negative (-) |
| Adipate | Positive (+) |
| Caprate | Positive (+) |
| Glucose | Positive (+) |
| Gluconate | Positive (+) |
| Lactose | Negative (-) |
| Maltose | Negative (-) |
| Malate | Positive (+) |
| Mannitol | Positive (+) |
| Mannose | Positive (+) |
Enzymatic Hydrolysis Tests
| Characteristics | Burkholderia pseudomallei |
| Acetate Utilization | Positive (+) |
| Arginine Dehydrolase | Positive (+) |
| Aesculin Hydrolysis | Negative (-) |
| Gelatinase | Positive (+) |
| PNPG | Negative (-) |
| ONPG (β-Galactosidase) | Negative (-) |
Burkholderia pseudomallei Associated Diseases
- Burkholderia pseudomallei is a causative agent of a deadly human disease ‘melioidosis’. This disease is mainly seen in humans and animals like pigs, goats, and sheep. This disease is transmitted by direct transmission of bacterium from soil or water to humans via skin cuts or wounds, ingestion, or inhalation route. Person-to-person or animal-to-person transmission is not reported yet. The death rate of melioidosis is very high – about 20 to 40% even with treatment. It is estimated that about 165,000 are infected with this disease per year and 89,000 of them can’t survive through it. (Limmathurotsakul D, Golding N, Dance DA, et al. , 2016)
- Most of the cases are reported in patients with diabetes, excessive alcohol use, chronic lung infections (cystic fibrosis and COPD), chronic renal diseases, hypertension, and immunocompromised conditions. Diabetes is the major risk factor for this disease because nearly 40% of melioidosis cases are reported in diabetic patients.
- Diverse clinical presentations are seen in melioidosis ranging from mild fever and skin rashes to severe conditions like pneumonia, bacteremia, abscesses in internal organs like the liver, spleen, kidney, and prostate, septic shock, osteomyelitis, septic arthritis, even CNS infection in rare cases.
- The clinical manifestations of melioidosis resemble those of tuberculosis so in many regions where melioidosis is not common, this disease is frequently misdiagnosed as tuberculosis. Also, cases of melioidosis misdiagnosed as aspergilloma based on cavitary lesions in chest X-rays and CT scans are also reported.
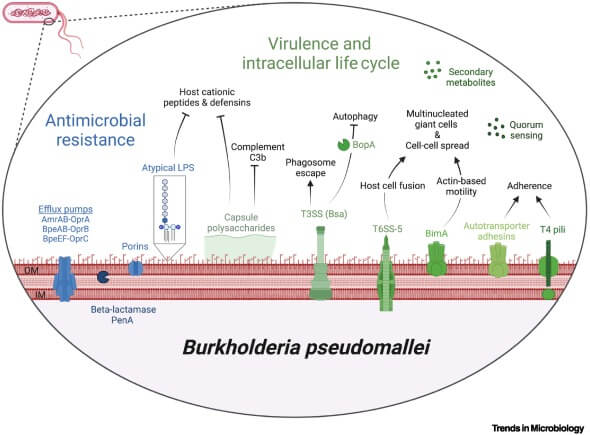
Virulence Factors of Burkholderia pseudomallei
B. pseudomallei exhibit numerous virulence factors, that help in disease pathogenesis, viz.
- Capsule and Pili: When the bacteria initially reach the host body, the capsule and pili aid in the initial epithelial or mucosal adhesion.
- Fimbriae: Type I fimbriae are present on the outer surface of the bacteria and these help in attachment with D-mannosylated sugars of surface glycoproteins of the host’s intestinal epithelium and macrophages.
- Autotransporters: These are outer membrane-anchored proteins that function as adhesins and help in bacterial adherence and invasion of human lung cells.
- Lipopolysaccharide (LPS): LPS, especially the O-antigen polysaccharide chain (O-PS) component of LPS, suppresses the host’s immune response in an early stage of infection.
- Oxidative stress-protecting factors like sigma factors, RpoS, and RpoE, catalase-peroxidase I enzyme, superoxide dismutase, putative ferritin DPS-family DNA binding protein, etc. are synthesized to protect against reactive oxygen species (ROS).
- Type III Secretion Systems (T3SS): These T3SS help bacteria escape endocytic vesicles and phagosomes and enter the host’s cytoplasm.
- Type VI Secretion Systems (T6SS): T6SS are important virulence factors that help in bacterial survival, cytotoxicity, multinucleated giant cell (MNGC) formation, etc.
- Bacterial phospholipases: These help in survival inside host cells, help in plaque formation, and induce cytotoxicity.
- Toxins: B. pseudomallei are capable of secreting a toxin called Burkholderia Lethal Factor 1 (BLF1).
- Burkholderia Intracellular Motility A (BimA) Protein: BimA protein helps in polymerization of host cell actin. This polymerization forms actin tails which are used by B. pseudomallei to propel themselves inside the host’s cytoplasm towards the host cell membrane.
- Efflux Pumps: Efflux pumps in their outer membrane play an important role in resistance against antibiotics such as aminoglycosides, colistins, and macrolides.
Pathogenesis of Burkholderia pseudomallei
- Burkholderia pseudomallei are soil bacteria and opportunistic pathogens that are mainly transmitted to human hosts via contaminated soils or water through 3 major routes viz. direct contact on susceptible skin, ingestion, or inhalation.
- Following exposure, they get attached to host cells and colonize there using capsules, pili, and adhesins. They begin to invade the host cells and get phagocytosed. They escape the killing mechanism within phagosomes and macrophages and rapidly escape into cytoplasm using a wide range of virulence factors like T3SS and multiply within the cytoplasm. They polymerize host actin fibers to form actin tails propel themselves to the host cell membrane and form MNGC which facilitates their spread in the host body/tissues. This causes damage to host cells which is further worsened by toxins and cytotoxic enzymes secreted by the infecting bacterium.
Disease Epidemiology of Burkholderia pseudomallei
Melioidosis is endemic in Northern Australia and Asian countries like Thailand, Vietnam, Malaysia, Singapore, and Southeast Asian regions like parts of India and Sri Lanka, southern China, Hong Kong, Taiwan, and Papua New Guinea. Other countries have also reported cases of melioidosis occasionally.
Laboratory Diagnosis and Identification of Burkholderia pseudomallei
Isolation of Burkholderia pseudomallei from infected sites is the ‘gold standard’ method to diagnose melioidosis. In the lab, several identification strategies are applied, based on availability, to identify B. pseudomallei.
- Colony characteristics and biochemical tests are common and easy techniques used for the identification of B. pseudomallei. For biochemical characterization, the API 20NE system is the most effective tool with its sensitivity and specificity of around 99%. The automated Vitek 1 system can also be used.
- Latex agglutination test is also available for its easy and rapid identification.
- ELISA using B. pseudomallei OPS antigens or other antigens to detect antibodies in a patient’s serum is also in practice for epidemiological surveys.
- Molecular techniques like PCR, gene sequencing, and blotting are superior to other techniques for precise identification.
Treatment Options and Antibiotic Resistance of Burkholderia pseudomallei
- Burkholderia pseudomallei is intrinsically resistant to penicillin, ampicillin, most early (1st and 2nd generation) cephalosporins, macrolides, rifamycins, aminoglycosides (gentamicin), and colistin (polymixins). These resistances are due to several antibiotic resistance mechanisms like bacterial membrane impermeability, efflux pump, enzymatic inactivation, drug sequestrations, altered antibiotic targets, enzymatic hydrolysis, etc.
- B. pseudomallei is susceptible to co-trimoxazole, amoxicillin-clavulanate, 3rd generation and newer cephalosporins, carbapenems, chloramphenicol, doxycycline, cefoperazone-sulbactam, etc.
- The treatment regimen is divided into two phases; the first intensive phase where IV antibiotics are administered followed by the second phase where oral antibiotics are prescribed. IV ceftazidime is the current drug of choice. Imipenem, meropenem, cefoperazone-sulbactam, and IV amoxicillin-clavulanate are the drugs of choice during the intensive phase. Co-trimoxazole is the preferred drug during the eradication phase. However, the choice of antibiotics must be made on the basis of antibiotic susceptibility testing and site and stage of infection.
- No vaccine is developed against this bacterium; however, co-trimoxazole is used as a prophylactic drug while working with the bacterium in high-risk areas or after post-exposure.
References
- Dance, David & Wuthiekanun, Vanaporn & Naigowit, P & White, N. (1989). Identification of Pseudomonas pseudomallei in clinical practice: Use of simple screening tests and API 20NE. Journal of clinical pathology. 42. 645-8. 10.1136/jcp.42.6.645.
- Zueter, AbdelRahman & Sawan, Hana & Zaiter, Amani & Harun, Azian. (2022). Current Protocols in Laboratory Diagnosis, Genotyping, and Treatment of Burkholderia pseudomallei. Clinical Microbiology Newsletter. 10.1016/j.clinmicnews.2022.01.003.
- Wuthiekanun, Vanaporn & Smith, M & Dance, David & Walsh, Amanda & Pitt, T & White, N. (1997). Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. Journal of medical microbiology. 45. 408-12. 10.1099/00222615-45-6-408.
- Britannica, The Editors of Encyclopaedia. “melioidosis”. Encyclopedia Britannica, 16 Sep. 2022, https://www.britannica.com/science/melioidosis. Accessed 31 August 2023.
- Prakash, A., Thavaselvam, D., Kumar, A., Kumar, A., Arora, S., Tiwari, S., Barua, A., & Sathyaseelan, K. (2014). Isolation, identification and characterization of Burkholderia pseudomallei from soil of coastal region of India. SpringerPlus, 3(1), 1-10. https://doi.org/10.1186/2193-1801-3-438
- Peacock SJ, Chieng G, Cheng AC, Dance DA, Amornchai P, Wongsuvan G, Teerawattanasook N, Chierakul W, Day NP, Wuthiekanun V. Comparison of Ashdown’s medium, Burkholderia cepacia medium, and Burkholderia pseudomallei selective agar for clinical isolation of Burkholderia pseudomallei. J Clin Microbiol. 2005 Oct;43(10):5359-61. doi: 10.1128/JCM.43.10.5359-5361.2005. PMID: 16208018; PMCID: PMC1248505.
- Shrestha, N., Adhikari, M., Pant, V. et al. Melioidosis: misdiagnosed in Nepal. BMC Infect Dis 19, 176 (2019). https://doi.org/10.1186/s12879-019-3793-x
- https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/burkholderia-pseudomallei.html
- O’Carroll MR, Kidd TJ, Coulter C, et al. Burkholderia pseudomallei: another emerging pathogen in cystic fibrosis. Thorax 2003;58:1087-1091.
- Limmathurotsakul D, Golding N, Dance DA, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008.
- Schweizer, H. P. (2012). Mechanisms of antibiotic resistance in Burkholderia pseudomallei: Implications for treatment of melioidosis. Future Microbiology, 7(12), 1389. https://doi.org/10.2217/fmb.12.116
- Amornchai, P., Chierakul, W., Wuthiekanun, V., Mahakhunkijcharoen, Y., Phetsouvanh, R., Currie, B. J., Newton, P. N., Chau, V., Wongratanacheewin, S., J. Day, N. P., & Peacock, S. J. (2007). Accuracy of Burkholderia pseudomallei Identification Using the API 20NE System and a Latex Agglutination Test. Journal of Clinical Microbiology, 45(11), 3774-3776. https://doi.org/10.1128/JCM.00935-07
- Virginio, C., Teixeira, M., Frota, C., Café, V., Rocha, M., & Sidrim, J.. (2006). Phenotypic characterization of three clinical isolates of Burkholderia pseudomallei in Ceará, Brazil. Memórias Do Instituto Oswaldo Cruz, 101(1), 95–97. https://doi.org/10.1590/S0074-02762006000100018
- Zakharova, I. B., Lopasteyskaya, Y. A., Toporkov, A. V., & Viktorov, D. V. (2018). Influence of Biochemical Features of Burkholderia pseudomallei Strains on Identification Reliability by Vitek 2 System. Journal of Global Infectious Diseases, 10(1), 7-10. https://doi.org/10.4103/jgid.jgid_39_17
- Howard, K., & J. Inglis, T. J. (2003). Novel Selective Medium for Isolation of Burkholderia pseudomallei. Journal of Clinical Microbiology, 41(7), 3312-3316. https://doi.org/10.1128/JCM.41.7.3312-3316.2003
- Chuaygud, T., Tungpradabkul, S., Sirisinha, S., Chua, K. L., & Utaisincharoen, P. (2008). A role of Burkholderia pseudomallei flagella as a virulent factor. Transactions of The Royal Society of Tropical Medicine and Hygiene, 102(Supplement_1), S140-S144. https://doi.org/10.1016/S0035-9203(08)70031-2
- Norris, M. H., Thu Tran, H. T., Walker, M. A., Bluhm, A. P., Zincke, D., Trung, T. T., Thi, N. V., Thi, N. P., Schweizer, H. P., Unger, F., Blackburn, J. K., & Thu Hang, N. T. (2020). Distribution of Serological Response to Burkholderia pseudomallei in Swine from Three Provinces of Vietnam. International Journal of Environmental Research and Public Health, 17(14). https://doi.org/10.3390/ijerph17145203
- Ekakoro, J. E., Lubega, A., Kayaga, E. B., Ndoboli, D., Bluhm, A. P., Wampande, E. M., Blackburn, J. K., Havas, K. A., & Norris, M. H. (2022). An Investigation of Burkholderia pseudomallei Seroprevalence in Market Pigs Slaughtered at Selected Pig Abattoirs in Uganda. Pathogens, 11(11), 1363. https://doi.org/10.3390/pathogens11111363
- Bzdyl, N. M., Moran, C. L., Bendo, J., & Sarkar-Tyson, M. (2022). Pathogenicity and virulence of Burkholderia pseudomallei. Virulence, 13(1), 1945-1965. https://doi.org/10.1080/21505594.2022.2139063
- https://www.health.ny.gov/guidance/oph/wadsworth/berkholderia.pdf
- https://my.clevelandclinic.org/health/diseases/24051-melioidosis
- https://www.cdc.gov/melioidosis/symptoms/index.html
- Melioidosis. (2023, August 30). In Wikipedia. https://en.wikipedia.org/wiki/Melioidosis
- https://netec.org/2022/09/08/burkholderia-pseudomallei-for-the-laboratory/
- https://www.sciencedirect.com/topics/medicine-and-dentistry/burkholderia-pseudomallei
