ATP (Adenosine Triphosphate) is commonly referred to as the “universal energy carrier” or “molecular currency” for energy transfer in cells. Every living thing relies on ATP generation as the main way to create energy. ATP production provides the energy for all intracellular activities.
ATP synthesis occurs through a process called oxidative phosphorylation that is carried out by five different protein complexes. Of these protein complexes, Complex V, also known as the ATP Synthase or ATPase, plays a crucial role in ATP production via the phosphorylation of adenosine diphosphate (ADP).
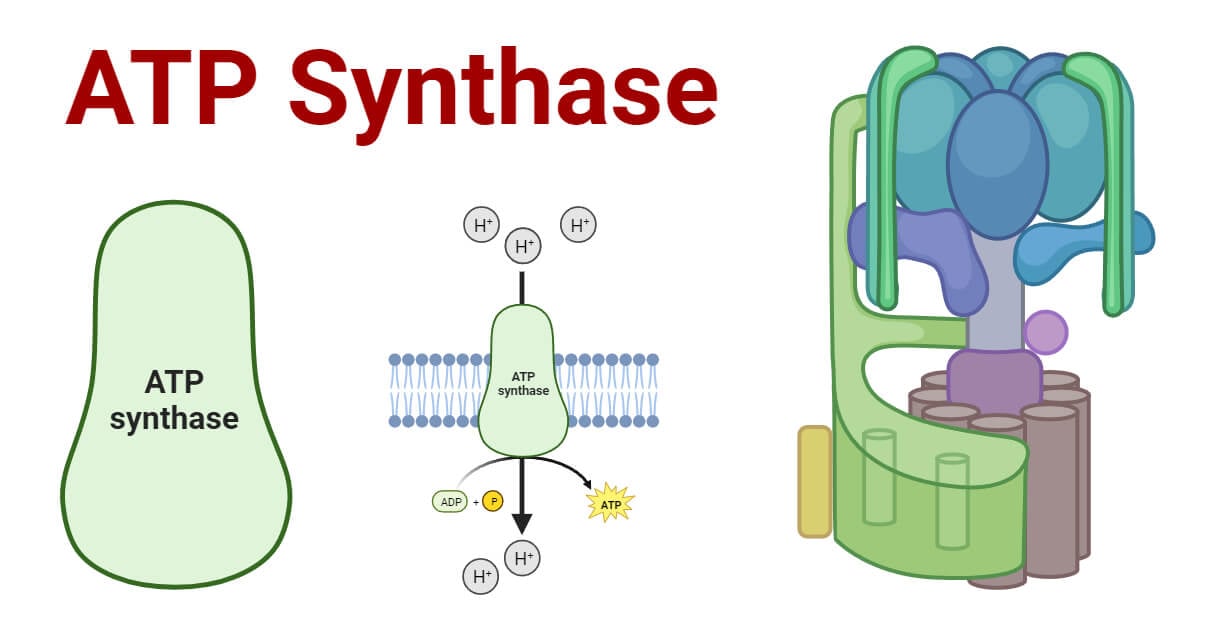
ATP synthase, also known as F1F0 ATPase or complex V, is the 5th subunit of the oxidative phosphorylation complex. It uses the energy from the electrochemical gradient of protons created by the electron transport chain to produce ATP from ADP.
ATP synthases constitute a diverse collection of enzymes that are conserved in various organisms. They are located in different cellular membranes: bacterial cytoplasmic membranes, thylakoid membranes in chloroplasts, and inner membranes of mitochondria.
Interesting Science Videos
Structure of ATP Synthase
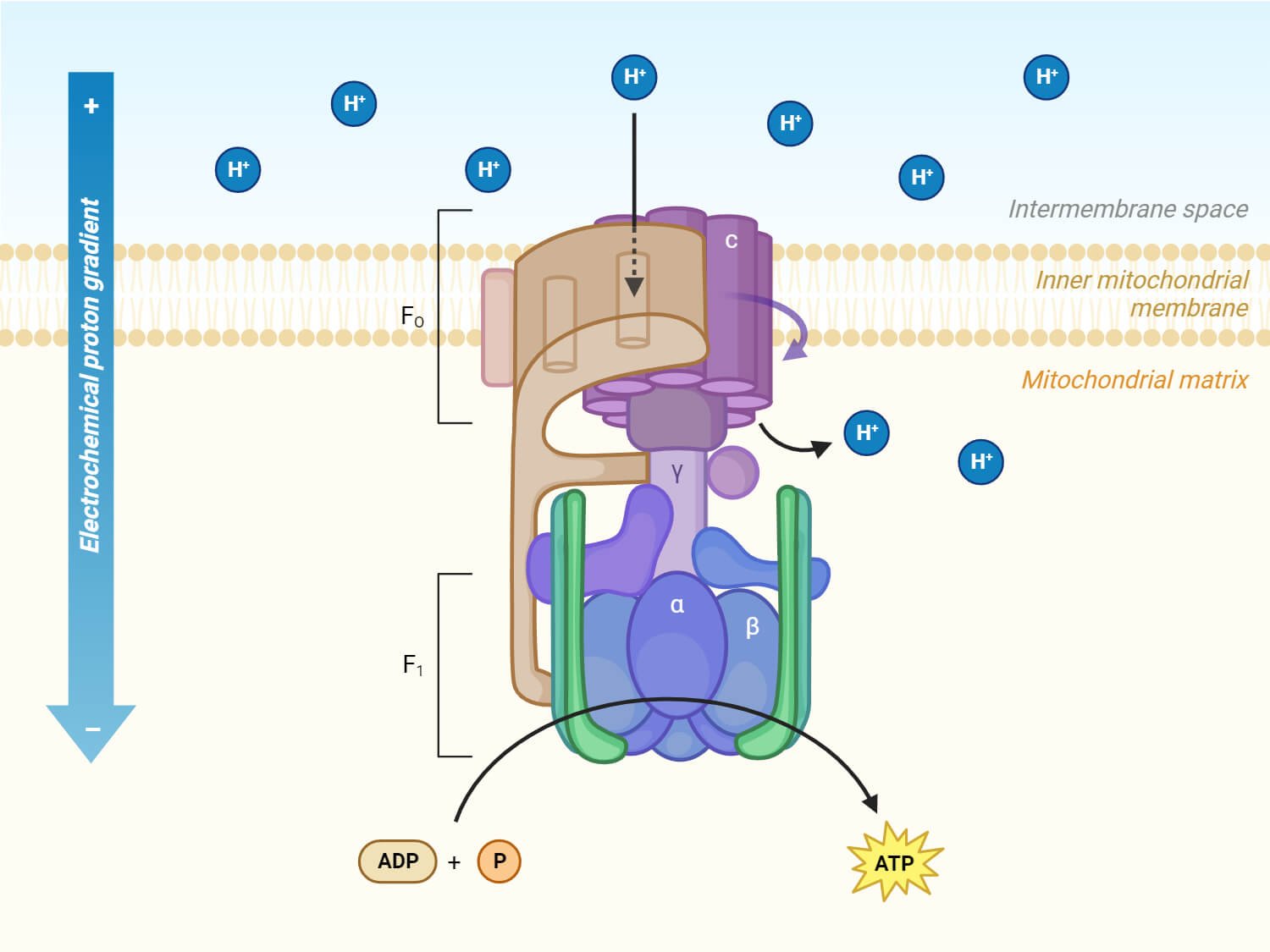
The structure of ATP synthase consists of two main protein components: the membrane-bound F0 portion which is attached to the inner mitochondrial membrane and the water-soluble F1 portion in the mitochondrial matrix.
F0 region
- F0 region is embedded in the cell’s membrane and is composed of three subunits, a, b, and c.
- This component acts as a proton channel, allowing the passage of protons across the membrane.
- In the F0 region, there is a ring composed of 9-12 c-subunits, arranged in a circular way. This ring is tightly linked to one a-subunit and two b-subunits of F0.
- The a-subunit acts as a mediator for proton transfer between the c-ring and the surrounding environment outside the cell.
- F0 is also bound to several other subunits within the F1 region of the synthase. The F0 and F1 domains are connected by the peripheral stalk.
F1 region
- F1 region is the catalytic part of the ATP synthase that is responsible for the synthesis of ATP using the energy generated by the proton flow through the F0 region.
- F1 region lies in the mitochondrial matrix and is made up of 5 subunits: α, β, γ, δ, and ε.
- It consists of three copies each of subunits α and β, along with one of each subunit γ, δ, and ε.
- The 3 copies of subunits α and β combine to form a hexameric ring structure called α3β3 which is the main part involved in the synthesis of ATP. Only the β subunits are capable of catalyzing the phosphorylation of ADP to produce ATP.
- The γ, δ, and ε subunits combine to form the central stalk that interacts with the c-ring of the F0 region.
Mechanism of ATP Synthesis
- ATP synthesis occurs through oxidative phosphorylation. During oxidative phosphorylation, electrons are transferred through a series of protein complexes in the electron transport chain (ETC).
- As these electrons move through the chain, protons (H+) are pumped across the inner mitochondrial membrane. This proton movement creates a proton gradient, also known as the proton motive force (PMF), with protons accumulating in the intermembrane space.
- ATP synthase utilizes this energy gradient to convert ADP and inorganic phosphate (Pi) into ATP.
- The chemiosmotic model, proposed by Peter Mitchell, explains how this proton motive force is used to synthesize ATP.
- The chemiosmotic model highlights the coupling of two processes: the flow of protons through ATP synthase and the phosphorylation of ADP to form ATP. This coupling is crucial for the conversion of energy stored in the proton-motive force into the chemical energy of ATP.
The ATP synthase mechanism can be explained in the following two processes:
Proton movement by F0
- The F0 region of ATP synthase plays a crucial role in the movement of protons across the inner mitochondrial membrane.
- The c-ring within F0 rotates, allowing protons to move across the membrane. This proton movement is driven by the electrochemical gradient generated by the electron transport chain.
- Subunit a of F0 is connected to the c-ring. Subunit a contains two channels that extend halfway into the subunit itself. These channels are pathways for protons to move within the enzyme.
- One of the half-channels opens to the intermembrane space, which is an area between the inner and outer membranes of a cell’s organelle, and the other half-channel opens to the matrix, which is the interior compartment of the organelle.
- The protons enter the half channel that faces the intermembrane space and bind to a specific amino acid on a subunit of the c-ring.
- As the c ring rotates, the bound protons move along with it. When they rotate to face the matrix half channel, they are released from the c subunit. This release is a result of the change in the c ring’s position.
- The rotation of the c ring also drives the movement of the γ subunit in the F1 region. This movement of the γ subunit, in turn, triggers changes in the conformation of the β subunits.
ATP Formation by F1
- The F1 region of ATP synthase is responsible for catalyzing the formation of ATP.
- The energy stored in the proton motive force also drives the rotation of the subunit γ within the F1 component.
- The rotation of the subunit γ causes conformational changes in α3β3 hexamer of F1, facilitating the binding of ADP and Pi to form ATP. This process is called rotary catalysis and is explained by the “binding-change” mechanism.
- The binding-change mechanism, first proposed by Boyer, suggests that the rotation of subunits within the ATP synthase complex facilitates ATP synthesis.
- The γ subunit rotates and interacts with different β subunits as it turns. The β subunits undergo conformational changes during ATP synthesis.
- During each 120° rotation of the γ subunit, it contacts a different β subunit, causing conformational changes in the β subunit.
- The three β subunits can exist in three forms: O (open), L (loose), and T (tight) forms.
- In the O state, the β subunit is in a state where ADP and Pi can either bind to it or be released from it.
- In the L state, the β subunit undergoes a conformational change that traps ADP and Pi within its structure. ADP and Pi that have bound to the β subunit in the O form are held in the L form.
- The T state of the β subunit is where the ATP synthesis occurs. In this conformation, ATP is synthesized from ADP and Pi without releasing the product. This ATP is released only when the T form shifts to the O form.
- With each complete rotation of the γ subunit, the β subunits shift through all three conformations, leading to the synthesis and release of ATP.
Significances of ATP Synthase
- ATP synthase is the primary mechanism by which cells generate ATP that is necessary for all cellular processes.
- ATP synthase maintains the electrochemical gradient generated by proton movement across the inner mitochondrial membrane. This gradient is useful for other processes, such as nutrient transport and oxidative phosphorylation.
- The structure and function of ATP synthase are highly conserved across different organisms, indicating its evolutionary significance.
- Understanding ATP synthase is important for studying and treating various diseases and disorders caused by dysfunctional ATP synthase.
References
- Ackerman, S. H., & Tzagoloff, A. (2005). Function, Structure, and Biogenesis of Mitochondrial ATP Synthase. Progress in Nucleic Acid Research and Molecular Biology, 95–133. doi:10.1016/s0079-6603(05)80003-0
- Fillingame, R. H., Angevine, C. M., & Dmitriev, O. Y. (2003). Mechanics of coupling proton movements toc-ring rotation in ATP synthase. FEBS Letters, 555(1), 29–34. doi:10.1016/s0014-5793(03)01101-3
- Jonckheere, A. I., M. Smeitink, J. A., & T. Rodenburg, R. J. (2012). Mitochondrial ATP synthase: Architecture, function and pathology. Journal of Inherited Metabolic Disease, 35(2), 211-225. https://doi.org/10.1007/s10545-011-9382-9
- Kubo, S., & Takada, S. (2022). Rotational Mechanism of FO Motor in the F-Type ATP Synthase Driven by the Proton Motive Force. Frontiers in Microbiology, 13, 872565. https://doi.org/10.3389/fmicb.2022.872565
- Neupane, Prashant, Bhuju, Sudina, Thapa, Nita and Bhattarai, Hitesh Kumar. “ATP Synthase: Structure, Function and Inhibition” Biomolecular Concepts, vol. 10, no. 1, 2019, pp. 1-10. https://doi.org/10.1515/bmc-2019-0001
- PDB-101: Molecule of the Month: ATP Synthase (rcsb.org)
- Whitehouse, D. G., May, B., & Moore, A. L. (2019). Respiratory Chain and ATP Synthase. Reference Module in Biomedical Sciences. doi:10.1016/b978-0-12-801238-3.95732-5
