- Aspergillus flavus is the second most common species of Aspergillus in humans after Aspergillus fumigatus.
- Aspergillus flavus is a mildly pathogenic, saprophytic mold, commonly known to cause diseases in plants such as grains, cereals, trees, and nuts. They cause opportunistic infections in crops.
- They infect the plants before and after harvesting when they are in storage rooms. In the pre-harvesting stage, the infection remains dormant until harvest time when it starts to cause yellowing in the infected parts of the plants.
- They are also known to produce mycotoxins which can cause poisoning in humans and animals.
- They also cause opportunistic infections such as aspergillosis in immunocompromised humans and animals.
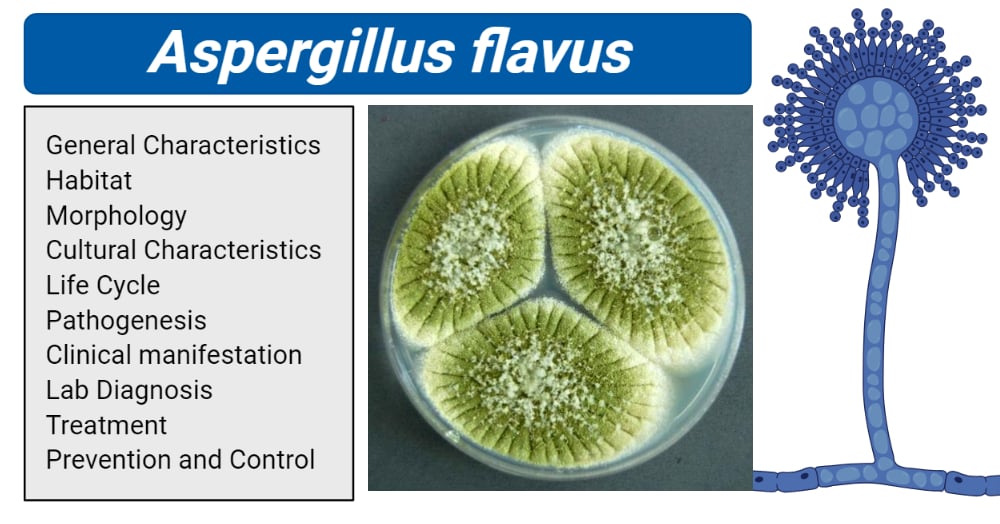
Image created using biorender.com. Image Source: http://fungi.myspecies.info/file-colorboxed/937
Interesting Science Videos
Habitat of Aspergillus flavus
- It is widespread globally and commonly found in soil.
- The fungus is present in the soil as conidia or sclerotia and in plant tissues as mycelia
- They are hosted by cereal grains, legumes, and tree nuts.
- They are thermotolerant fungi hence can survive in a wide range of surfaces than other fungi.
- They thrive in high moisture (hot and humid) environments
- They grow in a minimum temperature of 12 °C (54 °F) and a maximum temperature of 48 °C (118 °F), and an optimal growth temperature is 37 °C (98.6 °F).
- The optimum moisture level for the growth of Aspergillus flavus is 14%, however, these levels vary depending on the crops. Such as, for starchy cereals, they grow at moisture levels of 13-13.2% while for soybeans, at 11.5-11.9%.
- Sclerotia survive in the soil under severe environmental conditions and produce conidia and possibly ascospores based on recent data, leading to a population increase under hot and drought weather conditions.
Read also: Aspergillus fumigatus
Morphology of Aspergillus flavus
- The Aspergillus flavus group of fungi has a complex morphology that is classified based on the formation of sclerotia. Group I has L-strains whose sclerotia are greater than 400 μm in diameter and Group II has S strains with sclerotia less than 400 μm in diameter.
- They also have both sexual and asexual forms of reproduction.
- Asexual reproduction produces conidia spore and sclerotia while sexual reproduction produces sclerotia.
- Aspergillus flavus produces asexual spores known as conidia.
- The conidial spores are produced from the phialides on the conidiophore vesicles.
- The conidia spores have a thick mycelial mat that can be seen with unaided eyes and have a size of 3 to 6 µm.
- The conidiophore originates from the hyphal threads.
- The conidiophores are colorless and rough-textured.
- The phialides originating from the conidiophore are both uniseriate and biseriate
- Hyphae occur as thread-like septate branches that form mycelium.
- They branched hyphae have hyaline in each septae
- The hyphae are very tiny and can not be seen with naked eyes.
- Aspergillus flavus can also reproduce sexually producing ascospores contained within the sclerotia.
- Sexual reproduction occurs between two compatible strains with different vegetative forms that are cultured together.
Cultural characteristics of Aspergillus flavus
Sabouraud Dextrose Agar
- Aspergillus flavus produces white soft velvety colonies that turn yellowish-green, a pigment of the conidial spores.
Potato Dextrose Agar
- The conidia are characteristically green and sclerotia mass with a deep brown color.
Malt extract agar
- The mycelia are smooth which changes to olive green and colorless sclerotia.
Czapek yeast agar
- It produces colonies after 7 days of incubation at 25°C and 37°C.
- The colonies are velutinous, grey-blue-green, and uniseriate conidial heads.
The life cycle of Aspergillus flavus
- Aspergillus flavus lives through the winter season in soil, appearing as propagules on decaying matter as mycelia or a thick hard mass of mycelia know as sclerotia.
- The sclerotia germinate producing hyphae and asexual spores known as conidia.
- The conidia disperse into the air and the environment via insect (bugs)and wind type of pollinations.
- When the conidia land on grains and legumes, they infect them, through the silks of corn into the corn kernel.
- They grow producing conidiophore and conidia from the sclerotial surface.
- Some conidia may land on the surface of leaves which have been fed on by insects causing damage to the leaves, this is known as secondary inoculation.
- While, some spores may land in the soil via dispersing by rainwater, which then infects the oily plants such as peanuts and cotton seeds.
Pathogenesis and Clinical manifestations of Aspergillus flavus
- The pathogenicity of Aspergillus flavus in phenomenal of both plants and animals including humans. It causes infections in damaged plants and opportunistic infections in immunocompromised states.
Human Aspect of Aspergillus flavus infection
- The conidial spores of Aspergillus flavus bind to the lung cell basal lamina which leads to the development of invasive aspergillosis, enhanced by various proteins including fibronectin, laminin, type IV collagen, fibrinogen, complement, albumin, and surfactant proteins.
- Aspergillus flavus is majorly attracted to the fibrinogen proteins which allow adhesiveness to the basal lamina.
Aspergillosis
- Aspergillus flavus is the second leading cause of invasive and noninvasive aspergillosis and it is the most common cause of superficial infection.
- The clinical features of aspergillosis include allergic (extrinsic asthma, extrinsic allergic alveolitis, and allergic bronchopulmonary aspergillosis), pulmonary extrapulmonary colonization, and invasive infection (pulmonary and extrapulmonary).
- Other symptoms may include chronic granulomatous sinusitis, keratitis, cutaneous aspergillosis, wound infections, and osteomyelitis following trauma and fungal inoculation.
- Fungal sinusitis occurs due to deposition of the large spores in the upper respiratory tracts.
- Secondary transmission of fungal spores arise from infection via wounds and smoking contaminated plant material such as tobacco or marijuana.
- Nosocomial infections can occur during surgery, such as transplant patients.
Aflatoxicosis
- Toxicity is enhanced by the ingestion of the aflatoxins produced by the fungi.
- The sclerotia forms of the fungi are known to produce aflatoxins, commonly known as B1 and B2
- The S strain produces aflatoxin G1 and G2 which are not commonly produced by A.flavus.
- The L strain is more aggressive than S strain however it produces fewer aflatoxins, fewer sclerotia, with more acidity.
- The aflatoxins are carcinogenic and they cause aflatoxicosis
- Aflatoxicosis is associated with:
- vomiting, abdominal cramping, and pain, pulmonary edema, hemorrhaging, disruption of food digestion, poor abdominal absorption and metabolism, severe progressive effects may cause liver damage, liver cancer, mental impairment, coma, and death.
Carcinogenic effects of aflatoxins
- These are cancers arising from long-term exposure to aflatoxin B1. B1 is a potential hepatocarcinogen which induces tumors mainly in the liver, but also the kidney, lungs, and colon in humans and animals.
- Hepatocellular carcinoma (HCC), primary liver cancer, is associated with aflatoxin B1 consumption.
- Individuals with increased risks of developing aflatoxin associated Hepatocellular carcinoma (HCC) have chronic Hepatitis B and C, which synergically interacts with aflatoxin B1 increasing the development of HCC.
Plant pathologies
- Colonization of plants by Aspergillus flavus is enhanced by the mode of dispersion and damage by plant and insect eaters.
- The insects and plants provide a point of entry of the fungi into the plants while the insects and blowing wind, allows the spores to land on the damaged surfaces of these damaged plants and start to grow dormant until the plants are harvested and stored.
- During storage, the fungi start to germinate and spread within the crop and the surrounding crops.
- Colonization of plants by Aspergillus flavus forms powdery masses of yellowish-green spores on the upper surface and reddish-gold on the lower surface.
- In both grains and legumes, infection is minimized to small areas, and discoloration and dullness of affected areas are often seen. Growth is rapid and colonies appear downy or powdery in texture.
Aspergillus Ear Rot
- It is caused by aflatoxins produced by Aspergillus flavus.
- It is a powdery olive-green (yellow-green) mold that grows on the ears of corn and then turns brown as the masses age.
- Higher aflatoxin levels are associated with discolored, shriveled kernels that are often found near the tip of the ear.
- This infection is favored by hot dry conditions during pollination and during grain fill.
- Yellow-brown silks are most susceptible to infection.
- Spores landing on the silks germinate, rapidly grow down the silk and colonize the surface of the developing kernels.
- When the plant is maturing when the moisture levels drop, the fungi start to colonize the internal tissues and continue to grow until the moisture level decrease to <15%.
Laboratory Diagnosis for Aspergillus flavus
Microscopic Examination
- KOH wet mount – under the microscope, observe uncolored thick-walled conidiophores and rough or pitted vesicles. The vesicles are about 800-1200um in diameter, producing phialides. the phialides have uniseriate or biseriate or combined. The conidia are 250-450um with thin rough walls.
Culture observation
- Sabouraud Dextrose Agar– In the early days of growth (24-48 hours), the colonies are white with a soft velvety surface. After 4 days of growth, the colonies become raised and floccose at the center. On sporulation, the colonies appear yellowish-green because of the conidia color. Sclerotia are produced appearing white initially then they turn brown after 6 days of growth. Colonies are 55-70mmn in diameter
- Potato Dextrose Agar– they produce green conidia, with a dominated colony appearance. they are plain and flat at the edges and raised at the center and wrinkled cerebriform pattern. They also produce exudates that are colorless or brown. The sclerotia which are the compact mass of hardened fungal mycelia and deep brown in color. The colonies are encircled by a white border and a pale inner side.
- Malt extract agar- white Colonies vary in shape and size. The initial formation of smooth white mycelia which grows and produces olive and dark green conidia. Sclerotia are white and deep brown with colorless exudates at the center of the colonies.
- Czapek yeast agar– The mycelia are white, flat with large raised tufted wool of white mycelia. The colonies appear dry and exudated but no sclerotia are produced in CZA. No pigmentation observed do the colonies are uncolored. Some isolates produce velutinous, grey-blue-green, and uniseriate conidial heads.
Thin Layer Chromatography for the detection of aflatoxins and observed under the fluorescent Microscope.
Treatment of Aspergillus flavus infections
- A. flavus infection is typically treated with antifungal drugs such as amphotericin B, itraconazole, voriconazole, posaconazole, and caspofungin. In the case of the development of resistance, combined therapy can be applied.
- Use of essential oils such as Cinnamomum zeylanicum (cinnamon), Mentha piperita (peppermint), Ocimum basilicum (basil), Origanum vulgare (origanum), Teloxys ambrosioides (the flavoring herb epazote), Syzygium aromaticum (clove), and Thymus vulgaris (thyme) for storage of crops such as maize inhibits Aspergillus flavus growth.
- Thymol and o-methoxycinnamaldehyde significantly reduced maize grain contamination.
Prevention and Control
- Avoid exposure to fungal spores if you are allergic to fungi.
- Prophylactic treatment with amphotericin B and itraconazole to prevent for infection of Aspergillus flavus
- Removal of mold forming materials and food exposed to fungal toxins before ingestion.
References
- Alessandro C. P, 2009: Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus: Medical Mycology Review. Pages S261–S270
- Klich MA, 2007: Aspergillus flavus: the major producer of aflatoxin. Molecular Plant Pathology: 10.1111/j.1364-3703.2007.00436.x.
- Raymond J., Steven E, Screen, Bijan Shams-Pirzadeh. 2000: Lack of Host specialization in Aspergillus flavus: Applied and Environmental Microbiology. 10.1128/aem.66.1.320-324.2000
- Fausto A., Marcio L. R., Carolina C., 2019: The Still Underestimated Problem of Fungal Disease worldwide: Frontier of Microbiology: 2019/oo214
- McClenny N., Laboratory Detection and Identification of Aspergillus species by Microscopic observation and culture: The Traditional Approach. Medical Mycology. Volume 43, Pages S125–S128
- https://www.hindawi.com/journals/ijmicro/2017/5273893/
- https://www.microbiologyresearch.org/content/journal/micro/10.1099/mic.0.2007/007641-0?crawler=true
- https://crops.extension.iastate.edu/cropnews/2012/08/aspergillus-ear-rot-and-aflatoxin-production
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/aspergillus-flavus
- https://wiki.bugwood.org/Aspergillus_flavus
- http://www.biologydiscussion.com/fungi/aspergillus-habitat-reproduction-and-importance-ascomycotina/24000
- https://www.bustmold.com/resources/mold-library/aspergillus-flavus/
- http://agris.fao.org/agris-search/search.do?recordID=QY870002588
Sources
- 2% – https://www.researchgate.net/publication/264970927_AN_ASPERGILLOMA_CAUSED_BY_ASPERGILLUS_FLAVUS
- 2% – https://www.ncbi.nlm.nih.gov/pubmed/9709236
- 2% – https://core.ac.uk/download/pdf/38929216.pdf
- 1% – https://www.researchgate.net/publication/51070539_Aspergillus_flavus
- 1% – https://www.researchgate.net/publication/319973879_Morphological_Characterization_and_Determination_of_Aflatoxin-Production_Potentials_of_Aspergillus_flavus_Isolated_from_Maize_and_Soil_in_Kenya
- 1% – https://www.ncbi.nlm.nih.gov/pubmed/17526826
- 1% – https://www.farmprogress.com/story-check-fields-corn-ear-rot-aflatoxin-threat-9-62643
- 1% – https://www.deepdyve.com/lp/annual-reviews/aspergillus-flavus-8EEUowa0xX
- <1% – https://www.sciencedirect.com/topics/medicine-and-dentistry/extrinsic-asthma
- <1% – https://www.sciencedirect.com/topics/immunology-and-microbiology/stem-borer
- <1% – https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/aflatoxin
- <1% – https://www.researchgate.net/publication/51428186_Differences_in_pathogenicity_and_clinical_syndromes_due_to_Aspergillus_fumigatus_and_Aspergillus_flavus
- <1% – https://www.researchgate.net/publication/44635150_Aspergillus_flavus_The_major_producer_of_aflatoxin
- <1% – https://www.researchgate.net/publication/317118451_Morphological_and_Molecular_Diversity_of_Aspergillus_From_Corn_Grain_Used_as_Livestock_Feed
- <1% – https://www.researchgate.net/publication/291954339_Sclerotia_Formation_and_Toxin_Production_in_Large_Sclerotial_Aspergillus_flavus_Isolates_from_Kenya
- <1% – https://www.researchgate.net/publication/13676132_Interactions_of_Itraconazole_with_Amphotericin_B_in_the_Treatment_of_Murine_Invasive_Candidiasis
- <1% – https://www.nhs.uk/conditions/liver-cancer/
- <1% – https://www.ncbi.nlm.nih.gov/pubmed/5485087
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3629261/
- <1% – https://www.nature.com/articles/onc2010236
- <1% – https://www.bustmold.com/resources/mold-library/aspergillus-flavus/
- <1% – https://springerplus.springeropen.com/articles/10.1186/2193-1801-3-19
- <1% – https://moldpedia.com/aflatoxin
- <1% – https://academic.oup.com/mmy/article/56/suppl_1/S165/4925968
- <1% – https://academic.oup.com/mmy/article/44/Supplement_1/S9/1749057
- <1% – http://www.virtual-labs.leeds.ac.uk/brewing/isolation.php
