Apoptosis is a normal genetically programmed cell death where an aging cell at the end of its life cycle shrinks and its remaining fragments are phagocytosed without any inflammatory reaction.
The term apoptosis was first introduced in a paper in 1972 by Kerr, Wyllie, and Currie to describe a morphologically distinct type of cell death. It consists of a series of biochemical changes that lead to changes in the cell’s morphology or death. It results in the death of 50 to 70 billion cells per day in an average adult human being. It is also termed as ‘cellular suicide’ as cells undergo a highly regulated process for the programmed removal of cells from the body.
Interesting Science Videos
Why do cells undergo apoptosis?
- Most cells are provided with an in-built mechanism of apoptosis as a part of the cell cycle.
- This mechanism allows the body to get rid of unnecessary cells or infected cells.
- Apoptosis is considered a vital part of various processes including normal cell cycle, proper development and functioning of the immune system, embryonic development, and chemical-induced cell death.
- Apoptosis is a part of development as it is essential in the differentiation of a mass of tissue into various groups.
- Apoptosis occurs in cells that might have been infected with viruses or might even be cancerous. This process usually takes place when the cell detects defects in the DNA and is not able to repair it.
- Apoptosis is also an essential part of the immune system as it clears the pathogen-specific immune cells once the foreign particle is removed from the body.
- This also helps to remove the immune cells that might react against the body’s cells and cause autoimmune diseases.
- Another reason for apoptosis is to maintain homeostasis in the body by removing old cells to make space for the new ones.
Apoptosis mechanisms
The process of apoptosis is highly complex and sophisticated, involving an energy-dependent series of molecular events.
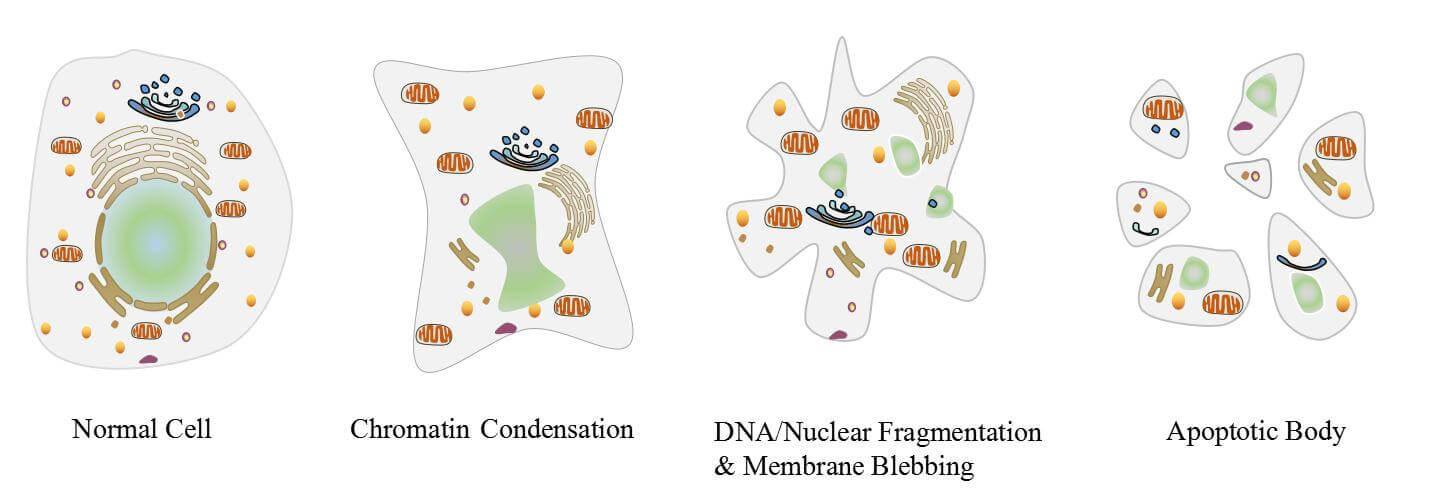
Image Source: CUSABIO.
Three different pathways work on different mechanisms to achieve apoptosis. All three of these pathways converge at the same terminal pathway, which results in the sequential degradation of cellular organelles.
1. Extrinsic or death receptor pathway
- The extrinsic pathway that initiates apoptosis involves transmembrane receptor-mediated interactions.
- These interactions take place between ligands and their corresponding death receptors that are all part of the tumor necrosis factor (TNF) family.
- All members of the TNF receptor family share a common cysteine-rich extracellular domain with about 80 amino acids called the “death domain”.
- The death domain plays a vital role in transmitting the death signal from the cell surface to the intracellular signaling pathways.
- The events or interactions that take place in the extrinsic phase of apoptosis involve two models; FasL/FasR and TNF-α/TNFR1 models, both of which include the clustering and binding of receptors and their ligands.
- Upon ligand binding, cytoplasmic adapter proteins are activated, which causes the receptors to exhibit death domains.
- The binding of FasL to FasR results in the activation of the adapter protein FADD whereas the binding of TNF ligand (TNF α) to TNF receptor (TNFR1) results in the binding of the adapter protein TRADD with activation of FADD and RIP.
- These events cause the dimerization of the death effector domain, causing FADD to bind with procaspase-8.
- As a result of the binding, a death-inducing signaling complex (DISC) is formed, resulting in the auto-catalytic activation of procaspase-8.
- Once caspase-8 is activated, the terminal phase or execution phase of apoptosis is triggered.
2. The intrinsic or mitochondrial pathway
- The intrinsic pathway that initiates apoptosis involves a series of non-receptor-mediated processes that produce intracellular signals and act directly on targets within the cell.
- This pathway involves mitochondrial-initiated events.
- The factors that initiate the intrinsic pathway produce intracellular signals that might act in either a positive or negative fashion.
- Negative signals include the absence of certain growth factors, cytokines, and hormones that can lead to failure of inhibition of death programs, thereby triggering apoptosis. In simple words, the withdrawal of factors causes loss of apoptotic suppression and subsequent activation of apoptosis.
- The factors that act positively include, radiation, toxins, hypoxia, hyperthermia, viral infections, free radicals, among others.
- All of these factors cause changes in the inner mitochondrial membrane that causes the opening of the mitochondrial permeability transition (MPT) pore and release of two main groups of pro-apoptotic proteins from the intermembrane space into the cytosol.
- The first group consists of cytochrome c that binds and activates Apoptotic protease-activating factor – 1(Apaf-1) as well as procaspase-9, forming a protein complex termed, apoptosome.
- The apoptosome cleaves the procaspase into the active form, caspase 9, which further cleaves and activates procaspase into the effector caspase 3.
- The first group also has other proteins like SMACs (second mitochondria-derived activator of caspases) and HtrA2/Omi that promote apoptosis by inhibiting the activity of IAPs (inhibitors of apoptosis proteins).
- The second group of pro-apoptotic proteins is released from the mitochondria during apoptosis, but this occurs as a part of the terminal phase after the cell has committed to die.
- These proteins translocate to the nucleus and cause DNA fragmentation and condensation of peripheral nuclear chromatin.
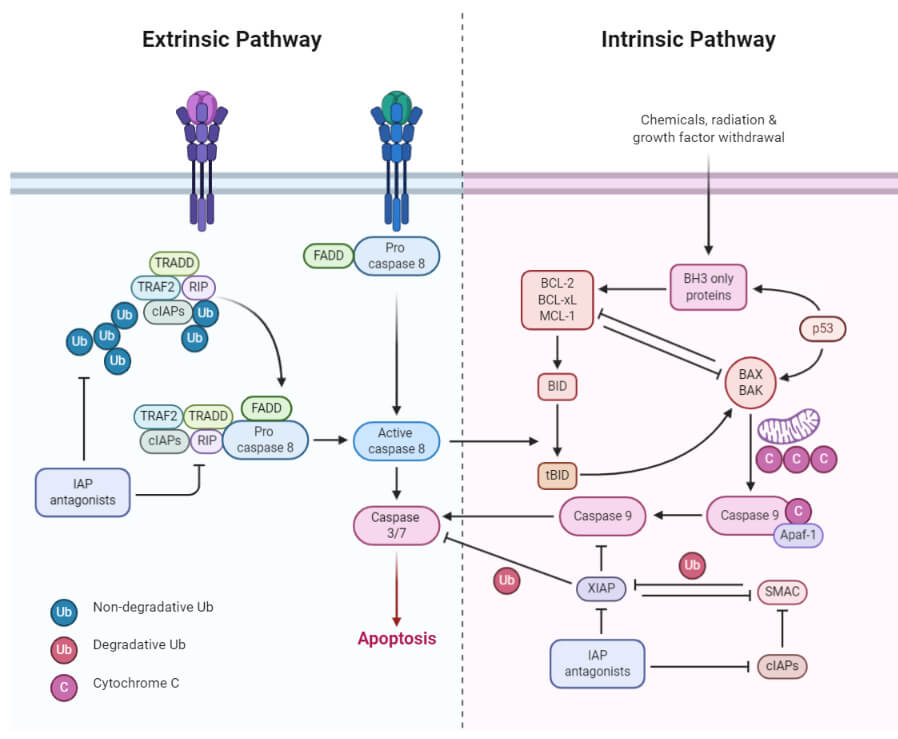
Figure: Extrinsic and Intrinsic Apoptosis. Image created using biorender.com.
3. Perforin/granzyme pathway
- Perforin/granzyme pathway is a novel pathway employed by cytotoxic T lymphocytes that exert their cytotoxic effects on tumor cells and virus-infected cells.
- This involves secretion of the transmembrane pore-forming molecule, perforin, with a subsequent release of cytoplasmic granules through the pore and towards the target cell.
- The granules consist of two crucial serine proteases; granzyme A and granzyme B that activate different proteins in the pathway.
- Granzyme B cleaves proteins at aspartate residues and therefore activates procaspase-10 and can cleave factors like ICAD (Inhibitor of Caspase Activated DNAse.
- It has also been observed that granzyme B can utilize the mitochondrial pathway for amplification of the death signal by induction of cytochrome c release.
- But granzyme B can also directly activate caspase-3. In this pathway, there is a direct induction of the execution phase of apoptosis.
- Granzyme A also has an essential role in cytotoxic T cell-induced apoptosis and activates caspase-independent pathways.
- As granzyme A reaches the cell, it activates DNA nicking by DNAse enzyme that prevents cancer through the induction of tumor cell apoptosis.
- Granzyme A protease cleaves the SET complex that inhibits the production of the DNAse enzyme.
- The proteins that make up the SET complex together protect chromatin and DNA structure. Thus, the inactivation of the SET complex by granzyme A contributes to apoptosis by blocking the maintenance of DNA and chromatin structure integrity.
4. Execution pathway
- Both the extrinsic and intrinsic pathways end at the point of the execution phase, considered the terminal pathway of apoptosis.
- This phase of apoptosis is initiated by the activation of various caspases that activate cytoplasmic endonucleases and proteases.
- The cytoplasmic endonucleases degrade the nuclear material, whereas the proteases degrade the nuclear and cytoskeletal proteins.
- Caspase-3 is the most important protein of the executioner caspases and is activated by any of the initiator caspases (caspase-8, caspase-9, or caspase-10).
- Caspase-3 precisely activates the endonuclease Caspase-activated DNase (CAD). CAD then causes chromatin condensation by degrading chromosomal DNA within the nuclei.
- Caspase-3 also causes cytoskeletal reorganization and disintegration of the cell into apoptotic bodies.
- Gelsolin, an actin-binding protein, is considered as one of the critical substrates of activated caspase-3. Caspase-3 cleaves gelsolin and the cleaved fragments of gelsolin, in turn, cleave actin filaments, resulting in disruption of the cytoskeleton and formation of apoptotic bodies.
- The later stages of apoptosis cause the appearance of phosphatidylserine on the outer leaflet of apoptotic cells.
- This facilitates noninflammatory phagocytic recognition, allowing for their early uptake and disposal.
- As the process takes place without the release of cellular components, no inflammatory response is elicited.
Inhibition of apoptosis
- Inhibition of apoptosis inhibits the cell death signaling pathways, which helps the tumor cells to escape apoptosis.
- Different groups of proteins act as negative regulators of apoptosis which are categorized as anti-apoptotic factors like IAPs and Bcl-2.
- IAP (Inhibitor of apoptosis) proteins represent a group of negative regulators of both caspases and cell death.
- IAP group in humans consists of 8 proteins, all of which have a characteristic BIR (Baculovirus IAP Repeat) domain that binds with the caspases and other proteins involved in apoptosis.
- Proteins like XIAP bind caspase-9 and caspase-3, thus inhibiting their activation and preventing apoptosis.
- Another factor, Bcl-2, governs mitochondrial membrane permeability and can be either pro-apoptotic or anti-apoptotic.
- The anti-apoptotic proteins include some proteins like Bcl-2, Bcl-x, and BAG that inhibit the release of cytochrome c and also modify the permeability of the mitochondrial membrane, thus inhibiting the intrinsic pathway of apoptosis.
- The ability of cells to escape apoptosis is the major cause of cancers like leukemia and multiple myeloma.
- Inhibition of apoptosis also induces loss of immune function by the immune system. The mutation of inhibition protein XIAP results in a rare genetically-mediated immunodeficiency.
Regulation of apoptosis
- Several proteins and genes regulate apoptosis. Specific families of proteins are involved in the regulation of apoptosis in various steps.
- Among all the factors, IAPs and Bcl-2 are two of the most important proteins involved that decide whether the apoptosis is going to complete or inhibit.
- The extrinsic pathway of apoptosis is inhibited by a protein called c-FLIP which will bind to FADD and caspase-8, rendering them ineffective.
- Another mechanism of apoptosis regulation in the extrinsic pathway involves a protein called Toso, which blocks Fas-induced apoptosis in T cells by inhibition of caspase-8 activation.
- In the intrinsic pathway, members of the Bcl-2 family play an important role in the regulation and control of the pathway.
- The Bcl-2 family of proteins controls mitochondrial membrane permeability, and the proteins can be either be pro-apoptotic or anti-apoptotic.
- The proteins of the Bcl-2 family regulate apoptosis by regulating the release of cytochrome c from the mitochondria via alteration of the mitochondrial membrane permeability.
- Proteins like Puma and Noxa are members of pro-apoptotic factors that facilitate the activation of apoptosis by preventing the action of anti-apoptotic factors.
- A group of proteins released from the mitochondria, Smac, promote apoptosis by inhibiting the action of IAPs in the mitochondrial pathway.
Apoptosis assays
- Because the process of apoptosis is regulated tightly at various points, it is possible to evaluate the activity of different proteins involved.
- It is essential to confirm the mechanism of cell death by two different assays as the process of apoptosis and necrosis might overlap.
- The first essay detects the initial events of apoptosis, whereas the second identifies the execution or terminal phase.
- Apoptosis assays have been divided into six different groups, which are:
1. Cytomorphological altercation
- The observation of hematoxylin and eosin-stained tissue sections with light microscopy allows the visualization of apoptotic cells.
- This method detects the cells in the later events of apoptosis, but the cells in the early stage of apoptosis are not recognized.
- Transmission electron microscopy (TEM) is the gold standard for the confirmation of apoptosis.
- In TEM, cells undergoing apoptosis reveals several structural characteristics. These characters include:
- electron-dense nucleus (marginalization of the nucleus in the early phase)
- nuclear fragmentation
- intact cell membrane even late in the cell disintegration phase
- disorganized cytoplasmic organelles
- large clear vacuoles
- phosphatidylserine at the cell surface.
- With the progression of apoptosis, these cells will lose the cell-to-cell adhesions and will separate from neighboring cells.
- Eventually, the cell will fragment into apoptotic bodies with intact cell membranes and will contain cytoplasmic organelles with or without nuclear fragments.
2. DNA fragmentation
- The DNA laddering technique is another method of detection of apoptosis that visualizes the products of endonuclease cleavage during apoptosis.
- This involves the extraction of DNA from a lysed cell homogenate separation by agarose gel electrophoresis.
- The resulting bands of DNA form a DNA ladder that can be used to detect apoptosis in tissues where the number of apoptotic cells is high.
- However, DNA fragmentation only occurs during the later stages of apoptosis, and thus this cannot detect cells in the early stages.
- Another method is the TUNEL (Terminal dUTP Nick End-Labeling) method which also detects the endonuclease cleavage products by enzymatically labeling the ends of DNA strands.
- Terminal transferase is used to attach dUTP to the 3′-end of the DNA fragments.
- The dUTP is then labeled with a variety of probes to allow detection by light microscopy, fluorescence microscopy, or flow cytometry.
- Although this technique is fast and can be conducted in a few hours, it might give false-positive results from necrotic cells.
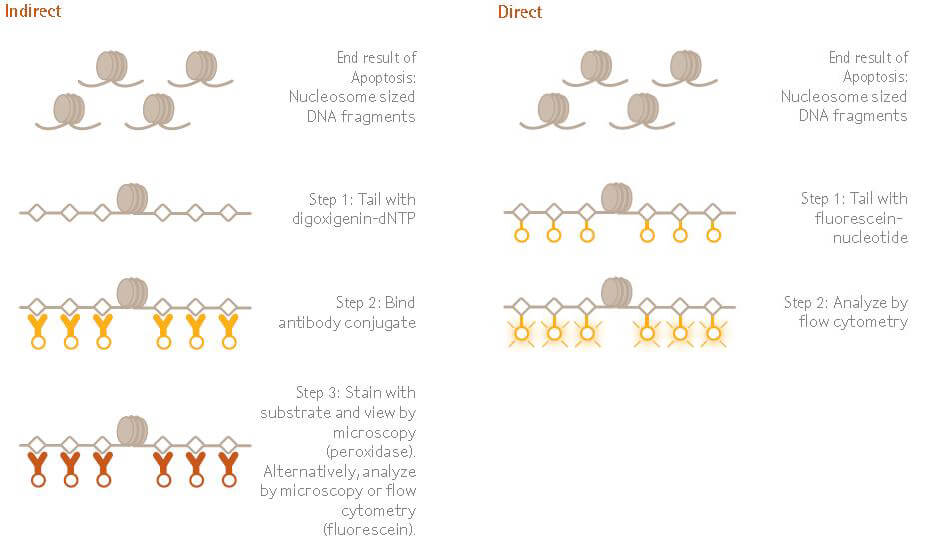
Figure: The ApopTag™ family of apoptosis kits examines DNA fragmentation by the TUNEL assay. The DNA strand breaks are detected by enzymatically labeling the free 3’-OH termini with modified nucleotides. These new DNA ends that are generated upon DNA fragmentation are typically localized in morphologically identifiable nuclei and apoptotic bodies.
Image Source: Merck KGaA.
3. Detection of caspases, cleaved substrate, regulators and inhibitors
- Various types of caspase activity assays are available that detect more than 13 known caspases involved in apoptosis.
- Some immunoassays can detect cleaved substrates such as PARP and known cell modifications such as phosphorylated histones.
- A variety of assays including western blot, immunoprecipitation, and immunohistochemistry can be used to detect caspase activation.
- Apoptosis PCR microarray is a comparatively new method that uses real-time PCR to indicate the expression of about 112 genes involved in apoptosis.
- The microarrays are designed to produce the expression profile of genes that encode essential receptors, ligands, intracellular regulators, and transcription factors involved in the regulation of programmed cell death.
- The genes involved in anti-apoptosis can also be assessed with this methodology.
- This technique can only provide an estimate of the number of apoptotic cells and thus has to be accompanied by other assays.
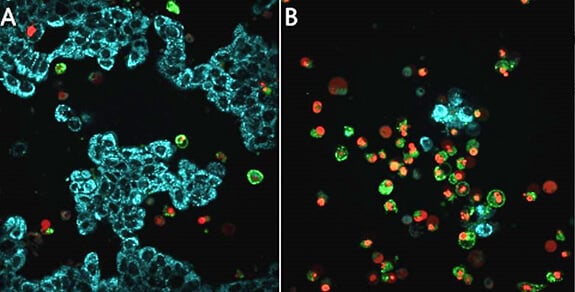
Figure: Live cell detection of mitochondrial membrane potential, caspase activity, and apoptosis in MCF-7 breast cancer cells. Untreated MCF-7 cells (86012803) (A) or treated with staurosporine (S5921) overnight to induce apoptosis (B), then stained with the BioTracker™ 405 Blue Mitochondria Dye (SCT135), BioTracker NucView® 530 Red Caspase-3 Dye (SCT105), and CF®488A Annexin V for 30 minutes at 37°C in cell culture medium with no wash. Healthy cells show mitochondrial staining (cyan), apoptotic cells lose blue staining (cyan), and show increased staining of nuclei (red) and CF®488A Annexin V staining of cellular membranes (green).
Image Source: Merck KGaA.
4. Membrane altercations
- The presence of phosphatidylserine residues on the outer plasma membrane of apoptotic cells can be detected via An-nexin V in tissues, embryos, or cultured cells.
- The apoptotic cells are first bound with FITC-labeled Annexin V and then visualized with fluorescent microscopy.
- This technique has a disadvantage as the membranes of necrotic cells might also be labeled. In order to avoid this, necrotic cells can be stained with membrane-impermeant nucleic acid dyes like propidium iodide and trypan blue to detect the loss of membrane integrity.
- In contrast, the membrane integrity of apoptotic cells can be detected by the absence of these dyes.
- The movement of phosphatidylserine to the outside of the cell membrane will also allow the transport of some dyes into the cell in a unidirectional manner.
- Thus, the cell might accumulate dye and shrinks in volume. As a result, the cell dye content becomes more concentrated and can also be visualized with light microscopy.
5. Detection of Apoptosis in Whole Mounts
- Dyes like acridine orange (AO), Nile blue sulfate (NBS), and neutral red (NR) can be used to visualize whole mounts of embryos or tissues.
- As these dyes are acidophilic, they tend to concentrate in areas of high lysosomal and phagocytotic activity.
- This technique should be associated with another assay as it cannot differentiate the apoptotic debris from the debris of microorganisms.
- These dyes, however, have certain disadvantages where acridine orange is mutagenic and toxic, and NBS and neutral red do not penetrate deep into the tissues and can be lost during preparation.
- Another dye, Lyso-Tracker Red, can be used with laser confocal microscopy to provide 3-dimensional imaging of apoptotic cells.
6. Mitochondrial assays
- Mitochondrial assays demonstrating cytochrome c release permit the detection of changes during the early stages of the intrinsic pathway.
- The Laser scanning confocal microscopy (LSCM) creates thin optical slices of living cells that are then used to monitor various mitochondrial events in intact single cells throughout a period of time.
- This technique measures parameters like mitochondrial permeability, depolarization of the inner mitochondrial membrane, mitochondrial redox status, Ca2+fluxes, and reactive oxygen species.
- However, these changes also occur during necrosis and thus cannot be used as detection of apoptosis exclusively.
- Other mitochondrial dyes that measure the redox potential or metabolic activity of the mitochondria in cells are also available. To determine the mechanism of apoptosis, however, a caspase detection assay should accompany this technique.
- Cytochrome c release from the mitochondria of living or fixed cells can also be assayed using fluorescence and electron microscopy.
- Pro-apoptotic or anti-apoptotic regulator proteins like Bax, Bid, and Bcl-2 can also be detected using fluorescence and confocal microscopy. However, the fluorescent protein tags might alter the interaction between the proteins and thus should be accompanied by other assays for confirmation.
Apoptosis significance/ Applications/ Roles
- Apoptosis is essential during development where many cells undergo programmed cell death, thus contributing to the formation of various tissues and organs from a single mass of tissue. It can even be used as an important determinant of fetal abnormalities.
- The regular removal of old cells from the body enables the body to produce new cells, helping maintain the cell population in the body. The inability to do so might result in dramatic consequences depending on the type of cells.
- Apoptosis helps in the removal of redundant and damaged cells from the body. At the same time, cells that are infected with a virus, and the ones that cannot be repaired are removed via apoptosis.
- Apoptosis is often used by the body’s immune system to check if the newly formed cells are self-destructive or not. If the immune cells are found to be destructive against the body’s cell, these are removed, preventing the possibility of autoimmune diseases.
- Apoptosis plays a critical role in the immune system of the body. Different cytotoxic cells like T lymphocytes are produced in advance during infection by foreign material. Once the invader is cleared from the body, the existing pathogen-specific immune cells are removed from the body via apoptosis.
- Apoptosis has also been observed in regulating the development of thymocytes and the shaping of T cells and the coordination of various immune responses.
- Apoptosis is also involved in the removal of about 50% of neurons produced during early embryonic development and in the formation of reproductive parts.
- Excess apoptosis might result in neurodegenerative diseases, and deficient apoptosis occurs in conditions like cancer and autoimmune diseases.
Video: Apoptosis (Khan Academy)
Examples of Apoptosis
Metamorphosis of the adult frog from tadpole
- Apoptosis is seen in frogs where many structures are destroyed and reabsorbed while the tadpole frog metamorphoses into an adult form.
- Tadpoles have gills, tail, and even fins which are removed from the body during its transformation into an adult frog.
- All of these structures are known to be destroyed by different mechanisms of apoptosis.
The nervous system in humans
- During the early development of the nervous system in the human embryo, a large number (almost 50%) of cells are removed via apoptosis.
- The exact reason behind the death of this many neurons is not entirely known. However, it has hypothesized that because the neurons tend to form complex connections, a larger number of cells are produced to ensure the efficiency of the process.
- As a result of this, a larger number of neurons will be produced, which are removed later to maintain the number of neurons in the nervous system.
Sloughing off of endometrium
- The process of removal of layers of cells in the endometrium of the uterus occurs by apoptosis.
- The periodic loss of cells in the endometrium and the corpus luteum forms the basis for menstruation, which is an important process in the female reproductive system.
Formation of hands and feet
- During the embryonic development of a multicellular organism, organs like hands and feet begin as a flat mass of tissue.
- As the development progresses, the tissue separates into individual fingers and toes where the cells connecting them are removed by apoptosis.
- This is an example of apoptosis involved in the formation and shaping of different organs from a singular mass of tissue.
Apoptosis and Cancer
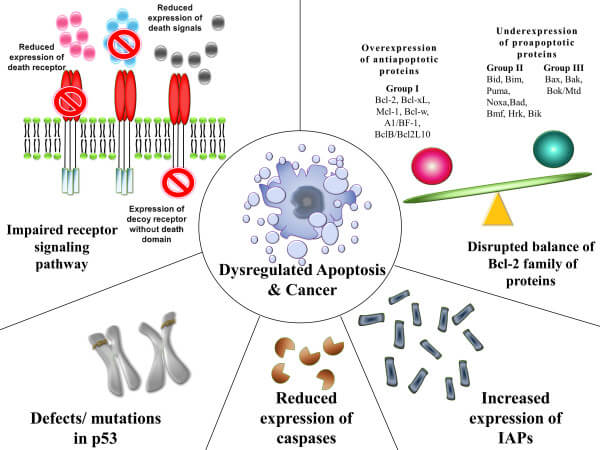
Figure: Mechanisms contributing to the evasion of apoptosis and carcinogenesis. Image Source: Rebecca SY Wong.
- Cancer is considered a result of several genetic changes in a normal cell, causing it to transform into a malignant one which requires the evasion of cell death as an essential change during the process.
- In simpler words, for a normal cell to be transformed into a malignant cell, it should escape the programmed cell death.
- Apoptosis is generally triggered by DNA damage which usually cannot be repaired by any mechanism in the cell.
- However, if the DNA damage results in damage to genes responsible for apoptosis, the process of apoptosis might not take place.
- Similarly, an imbalance in the pro-apoptotic and anti-apoptotic proteins in the cell might also inhibit the apoptosis of cells. The abnormal expression of the IAP family in pancreatic cancer cells has been observed to cause resistance to chemotherapy.
- Another reason might be reduced caspase function or reduced cell death signaling, which might also cause evasion of apoptosis by cells. An example of this is the downregulation of caspase-9 in patients with stage II colorectal cancer.
- Since apoptosis is considered as one of the causes of the induction of cancer in many cells, drugs, or treatment strategies to restore the apoptotic signaling pathways towards normality might have the potential to eliminate cancer cells.
- Thus a number of treatment strategies targeting the anti-apoptotic proteins and other factors that inhibit apoptosis have been developed.
Apoptosis in Plants
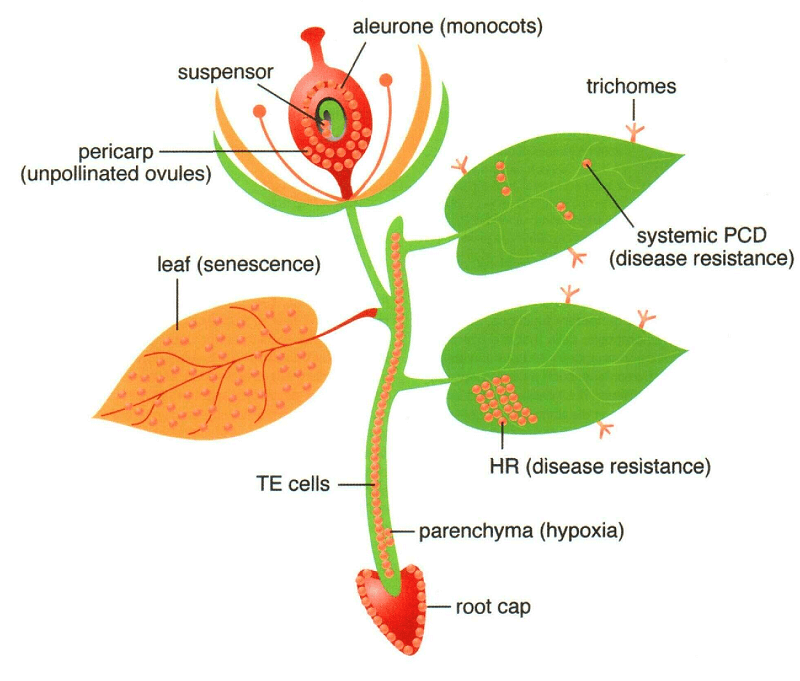
Figure: Sites of programmed cell death (PCD) in a Vascular Plant. Image Source: The American Society of Plant Biologists.
- The process and mechanism of apoptosis in plants have some similarities with the mechanism of programmed cell death in animals.
- However, some differences arise due to the presence of a cell wall in plants and the lack of an immune system that utilizes apoptosis for the removal of various particles.
- Programmed cell death is the preferred term while describing this process in plants.
- The process of programmed cell death in plants is controlled by cellular oxidative status, phytohormones, and DNA methylation.
- The mechanism of this process differs from that in animals as protease protein is used instead of caspases (in animals) that cause morphological changes in the plant cell.
- The activation of enzyme induces various morphological changes to the cell, causing the cell to break itself down and incorporate into a vacuole.
- The central vacuole then ruptures as the cell dies at the end of the programmed cell death.
- The morphological changes associated with programmed cell death in plants include:
- compaction and vacuolization of cytoplasm in the apoptotic cell
- specific fragmentation of cytoplasm and appearance of unique single-membrane vesicles containing active organelles in the vacuoles
- cessation of nuclear DNA synthesis
- condensation and accumulation of chromatin in the nucleus
- fragmentation of nuclear DNA in the nucleolus
- intensive synthesis of mitochondrial DNA in vacuolar vesicles.
Apoptosis vs Necrosis (17 Differences)
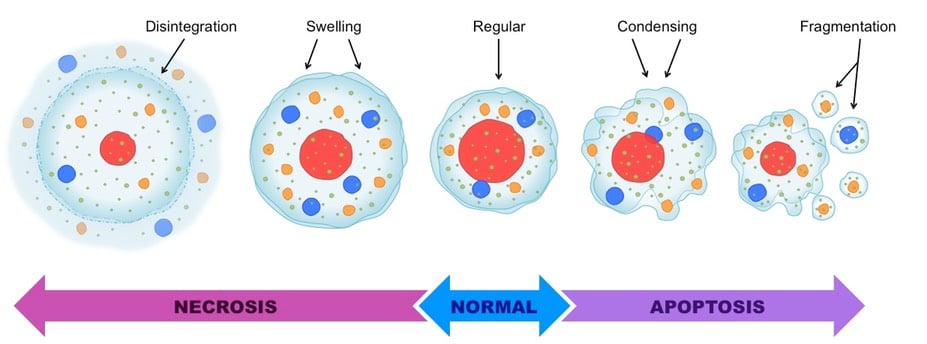
Image Source: BioNinja.
| Basis for Comparison | Apoptosis | Necrosis |
| Definition | Apoptosis is a normal genetically programmed cell death where an aging cell at the end of its life cycle shrinks and its remaining fragments are phagocytosed without any inflammatory reaction. | Necrosis is the premature death of a cell that occurs due to signals arising due to the presence of external agents like fungal or bacterial toxins. |
| Nature | Apoptosis is programmed and occurs as a regulated event. | Necrosis is not pre-planned and occurs at random if induced. |
| Control | It is genetically controlled | It is not genetically controlled. |
| Induced by | It is induced when the cell sends out signals of DNA damage or an imbalance of pro-apoptotic and anti-apoptotic proteins. | It is induced by the presence factors that are external like infection, trauma, and toxins. |
| Morphological changes | Membrane bleeding might occur, but the membrane integrity is maintained. | The cell ultimately loses its integrity. |
| Morphological changes | It begins with the shrinkage of the cytoplasm and condensation of the nucleus. | It begins with the swelling of the cytoplasm and mitochondria. |
| Morphological changes | Apoptosis ends with the fragmentation of the cell into smaller apoptotic bodies. | Necrosis ends with total cell lysis without the formation of membrane-bound vesicles. |
| Biochemical features | Some protein families tightly regulate the process. | The cell loses the regulation of ion homeostasis during necrosis. |
| Apoptosis is an active process that requires energy (ATP). | Necrosis is a passive process that doesn’t need energy. | |
| The DNA fragmentation during apoptosis occurs in a non-random way, forming mon- or oligonucleotide lengths. | The degradation of DNA during necrosis is random. | |
| A series of reactions of activation (cascade) of proteins takes place. | No cascade is formed during necrosis. | |
| Physiological changes | Apoptosis occurs in an individual cell and doesn’t affect the adjacent cells. | Necrosis affects a group of connected cells. |
| The phagocytosis after apoptosis occurs by either adjacent cells or macrophages. | The phagocytosis always takes place by macrophages. | |
| Apoptosis doesn’t induce any inflammatory response. | Necrosis causes a significant inflammatory response. | |
| Effects | Under normal conditions, apoptosis is beneficial. Problems arise when some factors cause either too many or too few cell deaths. | Necrosis always has detrimental effects. |
| pH changes | The pH of the cell becomes acidic during apoptosis. | The pH of the cell remains unchanged during necrosis. |
| Treatment | Apoptosis very rarely requires medical treatment. | Necrosis always requires medical treatment. |
References and Sources
- Tortora GJ and Derrickson B (2017). Principles of Physiology and Anatomy. Fifteenth Edition. John Wiley & Sons, Inc.
- Waugh A and Grant A. (2004) Anatomy and Physiology. Ninth Edition. Churchill Livingstone.
- Sharma S, Kaufmann T, Biswas S. Impact of inhibitor of apoptosis proteins on immune modulation and inflammation. Immunol Cell Biol. 2017;95(3):236-243. doi:10.1038/icb.2016.101
- Elmore S. (2007). Apoptosis: a review of programmed cell death. Toxicologic pathology, 35(4), 495–516. https://doi.org/10.1080/01926230701320337
- Berthelet, J., & Dubrez, L. (2013). Regulation of Apoptosis by Inhibitors of Apoptosis (IAPs). Cells, 2(1), 163–187. https://doi.org/10.3390/cells2010163
- Wong R. S. (2011). Apoptosis in cancer: from pathogenesis to treatment. Journal of experimental & clinical cancer research : CR, 30(1), 87. https://doi.org/10.1186/1756-9966-30-87
- Vanyushin BF, Bakeeva LE, Zamyatnina VA, Aleksandrushkina NI. Apoptosis in plants: specific features of plant apoptotic cells and effect of various factors and agents. Int Rev Cytol. 2004;233:135-179. doi:10.1016/S0074-7696(04)33004-4
- https://www.biology-pages.info/A/Apoptosis.html
- https://biologydictionary.net/apoptosis/
- https://www.diffen.com/difference/Apoptosis_vs_Necrosis
- https://www.uccs.edu/Documents/rmelamed/apoptosis_003_004.pdf

thank you so much…. this is perfect
Just superb… This crisp form of notes on apoptosis cannot even be found in any reference book or article.
Very good