Humans have used fermentation since ancient times to produce alcoholic beverages, non-alcoholic fermented foods, and many other useful products. Fermentation is a metabolic process in which organisms convert carbohydrates, like starch or sugars, into either alcohol or acids. This process is an essential alternative way to generate energy in the form of ATP for many microorganisms in the absence of oxygen, hence it is an anaerobic pathway.
Louis Pasteur, a French chemist and microbiologist, was the first scientist to study fermentation. In 1857, he showed that yeasts perform ethanol fermentation without oxygen. When yeast cells were absent, fermentation didn’t happen. In 1887, Eduard Buchner discovered that yeast extract can drive fermentation without free cells, laying the groundwork for understanding the enzymatic reactions in converting sugar to ethanol and CO2.
Interesting Science Videos
What is Alcohol Fermentation?
Alcohol fermentation, also known as ethanol fermentation, is a process in which sugars like glucose are converted into alcohol and carbon dioxide.
- In addition to ethanol, other products of alcoholic fermentation include higher alcohols, esters, glycerol, succinic acid, diacetyl, acetoin, and 2,3-butanediol.
- This process is mainly used for manufacturing alcoholic beverages. It is also used in breadmaking. Most of the ethanol evaporates during baking and contributes to the flavor and aroma of bread. CO2 released during the fermentation makes breads fluffy.
- Many microorganisms, including yeasts and bacteria such as Zymomonas mobilis, can produce ethanol as the major fermentation product from carbohydrates.
- Alcoholic fermentation is mainly carried out by yeast. Although other microorganisms can also make alcoholic beverages, yeasts are the primary fermenters in the production of alcoholic drinks.
- Saccharomyces cerevisiae is the most common kind of yeast used in fermentation. It converts the sugars from different sources, e.g., grapes for wine and barley for beer, into alcohol and carbon dioxide. Specific beverage requires specific yeast strains and methods to obtain the desired characteristics.
Process of alcohol fermentation
The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide.
The production of ethanol from glucose can be represented by the following chemical equation:
C6H12O6 (glucose) → 2 C2H5OH (ethanol) + 2 CO2 (carbon dioxide)
Alcohol fermentation occurs in two major stages:
1. Glycolysis
Glycolysis is the first stage of alcohol fermentation which involves the breakdown of glucose into 2 pyruvate molecules. This pathway is the initial stage of carbohydrate breakdown in most organisms.
Glycolysis consists of a series of 11 chemical reactions and its primary function is to break down sugars and release energy in the form of ATP.
The glycolytic process can be summarized by the following equation:
C6H12O6 + 2 ADP + 2 Pi (inorganic phosphate) + 2 NAD+ → 2 CH3COCOO− (Pyruvate) + 2 ATP + 2 NADH + 2 H2O + 2 H+
The overall products of these reactions are two pyruvate molecules, two NADH, and two molecules of ATP. The pyruvate molecules are further processed in the absence of oxygen to form ethanol.
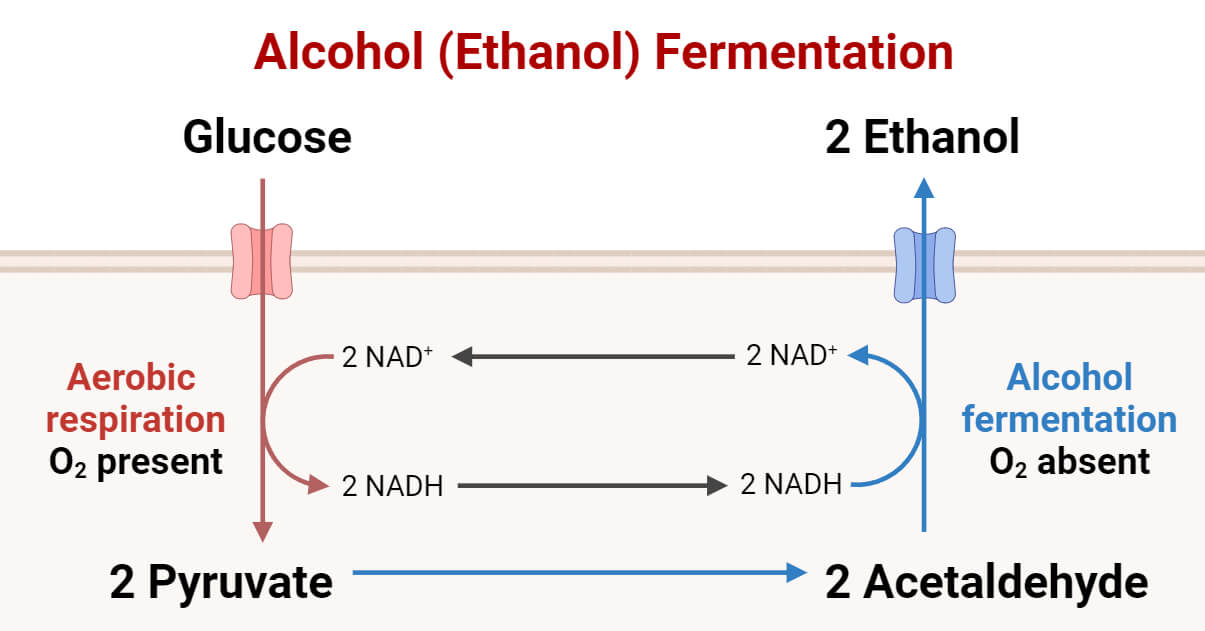
2. Pyruvate to Ethanol Conversion
During the second stage of alcohol fermentation, pyruvate molecules are converted into 2 molecules of ethanol and carbon dioxide. This conversion of pyruvate to ethanol takes place in two further steps:
In the first step, the carboxyl group of pyruvates is removed and released in the form of CO2. The product of this reaction is acetaldehyde. This reaction is catalyzed by pyruvate decarboxylase enzyme.
CH3COCOO− + H+ → CH3CHO (acetaldehyde) + CO2
In the second step, the acetaldehyde molecule is reduced. One molecule of NADH passes its electrons to acetaldehyde, forming ethanol. The NAD molecule is regenerated during this process. This reaction is catalyzed by alcohol dehydrogenase.
CH3CHO + NADH + H+ → C2H5OH (ethanol) + NAD+
Video on Alcohol Fermentation (Ethanol Fermentation)
Alcoholic Beverages
Alcoholic beverages include a wide variety of products that result from fermentation and distillation of ingredients such as fruits, grains, and sugars. These beverages include wine, beer, and distilled liquors. In addition to these popular alcoholic beverages, there are many other lesser-known locally consumed drinks around the world.
- Wine fermentation: Wine is produced by the fermentation of fruit. Grapes are the most commonly fermented fruit. S. cerevisiae is the most commonly used strain during wine production. Yeasts use the sugar in the grapes or other fruits and convert it to ethanol and CO2. The alcohol content of wine is about 6-14 %.
- Beer fermentation: The most widely consumed alcoholic beverage worldwide is beer. Malted grains (such as barley) are used as raw materials to produce beer. Yeast cells convert starch in these grains into ethanol and CO2. Two main species of yeast are used in brewing: S. cerevisiae is a top-fermenting yeast used to produce ales whereas S. pastorianus is a bottom-fermenting yeast used in the brewing of lagers. The alcohol content of beer is about 4-6 %.
Uses of Alcohol Fermentation
- Alcohol fermentation is mainly used in the production of alcoholic beverages like beer, wine, and spirits. Yeasts ferment sugars from various sources, converting them into ethanol and carbon dioxide.
- Alcohol fermentation also plays a crucial role in baking. It enhances the flavor and aroma of bread. The carbon dioxide released from fermentation causes the dough to rise and contributes to the fluffy texture of bread.
- Alcohol fermentation is also used to produce biofuels such as bioethanol which is a renewable source of energy used as an alternative to fossil.
- The process of alcohol fermentation is also used in various industries to produce various chemicals and pharmaceuticals such as organic acids and antibiotics.
- Alcohol fermentation is also used in the food and perfume industries to create flavors and fragrances.
- Alcohol fermentation also has traditional and cultural significance. Alcoholic drinks are included in several religious rituals in many societies.
References
- 1.12: Fermentation – Biology LibreTexts
- Alba-Lois, L. & Segal-Kischinevzky, C. (2010) Beer & Wine Makers. Nature Education 3(9):17
- Alcohol Fermentation | Facts, Process & Reaction Types (alevelbiology.co.uk)
- De Vasconcelos, J. N. (2015). Ethanol Fermentation. Sugarcane, 311–340. doi:10.1016/b978-0-12-802239-9.00015-3
- Dussap, C.-G., & Poughon, L. (2017). Microbiology of Alcoholic Fermentation. Current Developments in Biotechnology and Bioengineering, 263–279. doi:10.1016/b978-0-444-63666-9.00010-8
- Helmenstine, Anne Marie, Ph.D. “What Is Fermentation? Definition and Examples.” ThoughtCo, Sep. 7, 2021, thoughtco.com/what-is-fermentation-608199.
- History and Biochemistry of Fermented Foods (rockefeller.edu)
- Maicas, S. (2020). The Role of Yeasts in Fermentation Processes. Microorganisms, 8(8). https://doi.org/10.3390/microorganisms8081142
- Zamora, F. (2009). Biochemistry of Alcoholic Fermentation. In: Moreno-Arribas, M.V., Polo, M.C. (eds) Wine Chemistry and Biochemistry. Springer, New York, NY. https://doi.org/10.1007/978-0-387-74118-5_1

