Interesting Science Videos
Introduction
- Fermentation is one of the ancient food processing technologies.
- Fermentation is defined as a process in which chemical changes occur in an organic substrate through the action of enzymes produced by microorganisms.
- For example, yeast enzymes convert sugars and starches into alcohol, while proteins are converted to peptides/amino acids.
- Fermentation takes place in the lack of oxygen that produces ATP (energy).
- It turns NADH and pyruvate produced in the glycolysis step into NAD+ and various small molecules depending on the type of fermentation.
- The fermenting microorganisms mainly involve L.A.B. like Enterococcus, Streptococcus, Leuconostoc, Lactobacillus, and Pediococcus and yeasts and molds like Debaryomyces, Kluyveromyces, Saccharomyces, Geotrichium, Mucor, Penicillium, and Rhizopus species.
Principle of fermentation
- The main principle of fermentation is to derive energy from carbohydrates in the absence of oxygen.
- Glucose is first partially oxidized to pyruvate by glycolysis.
- Then pyruvate is converted to alcohol or acid along with regeneration of NAD+ which can take part in glycolysis to produce more ATP.
- Fermentation yields only about 5% of the energy obtained by aerobic respiration.
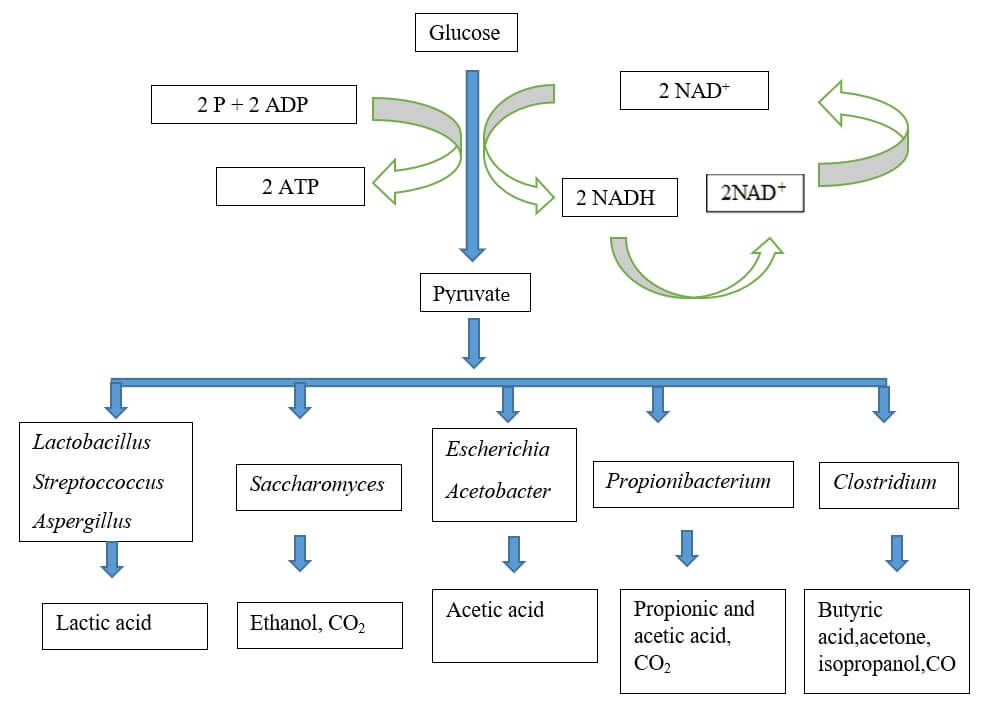
Flowchart: Generalized pathways for the production of some fermentation end products from glucose by various organisms.
- Fermentation is an anaerobic biochemical process that is used for the production of energy from the partial oxidation of glucose or other carbon sources.
- The oxidation of the substrate, which occurs through the Embden–Meyerhoff (EMP) or Entner–Doudoroff(ED) pathways, results in the production of pyruvate, ATP, and NAD (P) H.
- In the absence of external electron acceptors, the pyruvate undergoes reduction with the regeneration of NAD+(P).
- This step is essential for the fermentation process to progress and it leads to the production of products (ethanol and organic acids).
- ATP is the main product of fermentation, and it is generated by phosphorylation at the substrate level.
- NADH is then re-oxidized, reverting to NAD+ in the second phase of fermentation that reduces pyruvate to the fermentation product, such as ethanol and lactate.
- For example, in the fermentation of glucose by Streptococcus lactis, the pyruvate is converted to lactic acid to reform NAD+ coenzymes so two ATP molecules are produced
- In yeasts like Saccharomyces, when pyruvate is converted to ethyl alcohol (ethanol), NAD+ is reformed.
Types of fermentation
1. Lactic acid homofermentation
Glucose → Lactic acid
- Homolactic fermentation is carried out by bacteria belonging to the genera Lactococcus, Enterococcus, Streptococcus, and Pediococcus, and by some species of the genus Lactobacillus.
- Homofermentative LAB ferment glucose to lactic acid.
- Lactococcus spp. is used in the dairy starter culture.
2. Lactic acid heterofermentation
Glucose → Lactic acid + Acetic acid + Ethyl alcohol + 2CO2 + H2O
- Heterolactic fermentation is carried out by bacteria of the genera Leuconostoc, Oenococcus, and Weissella, and by heterofermentative lactobacilli.
- Heterofermentative LAB ferment glucose with lactic acid, ethanol/acetic acid, and carbon dioxide (CO2 ) as by-products.
3. Propionic acid fermentation
Glucose → Lactic acid + Propionic acid + Acetic acid + CO2 + H2O
- Propionic acid fermentation is carried out by several bacteria that belong to the genus Propionibacterium and the species Clostridium propionicum.
- During propionic acid fermentation, both sugar and lactate can be used as the initial substrate.
- When sugar is available, these bacteria use the EMP pathway to produce pyruvate; the pyruvate is carboxylated to oxaloacetate and then reduced to propionate via malate, fumarate, and succinate.
- The other end products of propionic fermentation are acetic acid and CO2.
4. Diacetyl and 2,3-butylene glycol fermentation
Diacetyl
↑
Citric acid→ Pyruvic acid + Acetylmethylcarbon
↓
2,3-Butylene glycol
- Butanediol fermentation is carried out by members of the genera Enterobacter, Erwinia, Hafnia, Klebsiella, and Serratia.
- The reactions that lead to the production of 2,3-butanediol involve a double decarboxylation step.
5. Alcoholic fermentation
Glucose → Ethyl alcohol
- Alcoholic fermentation is the best known of the fermentation processes.
- It is carried out by yeasts and some other fungi and bacteria.
- The first step of the alcoholic fermentation pathway involves pyruvate, which is formed by yeast via the EMP pathway, while it is obtained through the ED pathway in the case of Zymomonas (bacteria).
- The redox balance of alcoholic fermentation is achieved by the regeneration of NAD+ during the reduction of acetaldehyde to ethanol.
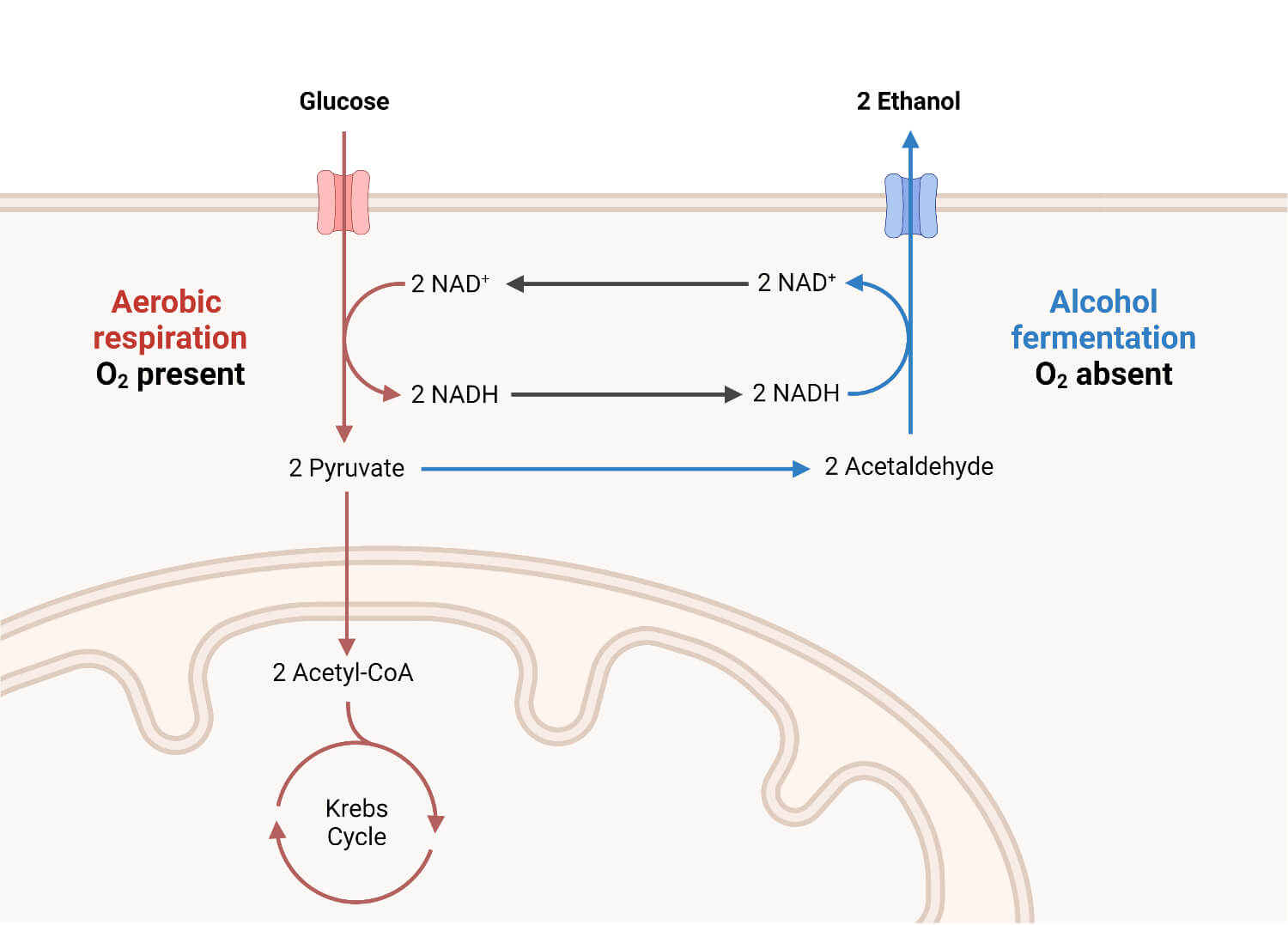
Figure: Alcohol Fermentation. Created with biorender.com.
6. Butyric acid fermentation
Glucose → Acetic acid + Butyric acid
- Butyric acid fermentation is characteristic of several obligate anaerobic bacteria that mainly belong to the genus Clostridium.
- Pyruvate is in turn oxidized to acetyl-CoA, with the production of CO2 and H2.
- Part of the acetyl-CoA is converted into acetic acid, with ATP production.
- Some bacteria, such as Clostridium acetobutylicum, produce fewer acids and more neutral products, thus carrying out acetone butanol fermentation.
Applications of fermentation
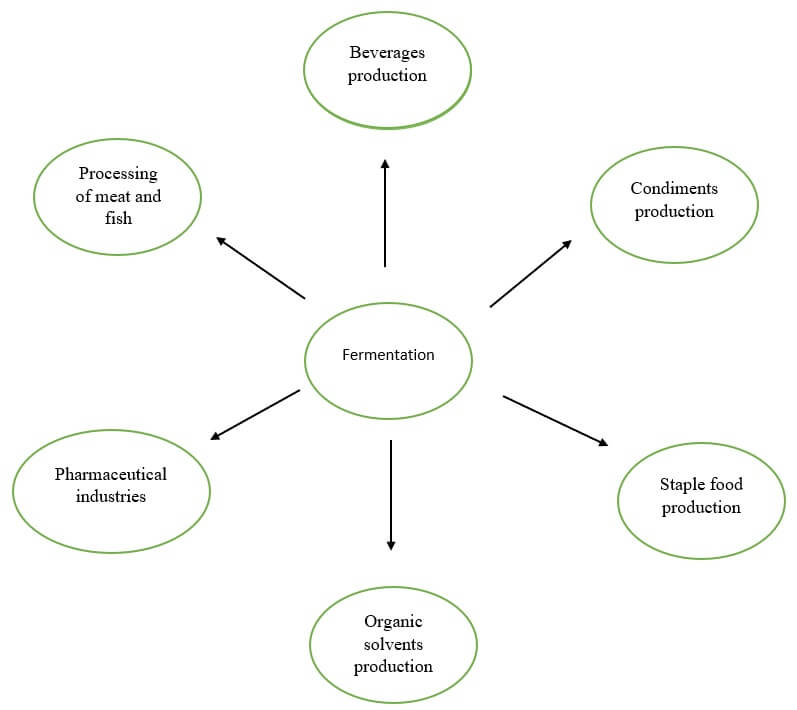
Figure: Application of fermentation.
Application in medicine
- Production of antibiotics
- Production of insulin
- Production of growth hormones
- Production of vaccines
- Production of interferon
Application in the food industry
- Production of fermented foods as cheese, wine, beer, and bread to high-value products
- Food grade bio preservatives
- Functional foods/Neutraceuticals
- Production of single-cell protein
Other Applications
- It is also used for waste management such as biofuels production (biodiesels, bioethanol, butanol, biohydrogen, etc).
- It is also used to produce bio-surfactant, polymers production such as bacterial cellulose production.
- Development of bioremediation processes (involving microbes or their isolated enzymes) for soils and wastewater treatments.
Limitations of fermentation
- Low scale production that requires high cost and high energy
- Possibilities of contamination.
- Natural variations over time
- the product is impure which needs further treatment
- the undesirable and unexpected end product
- the undesirable microbes grow and multiply and desirable microbes died.
References
- Admassie, M. (2018). A Review on Food Fermentation and the Biotechnology of Lactic Acid Bacteria. World Journal of Food Science and Technology, 2(1), 19. https://doi.org/10.11648/j.wjfst.20180201.13
- Ciani, M., Comitini, F., & Mannazzu, I. (2018). Fermentation. Encyclopedia of Ecology, June, 310–321. https://doi.org/10.1016/B978-0-12-409548-9.00693-X
- Ghosh, B., Bhattacharya, D., & Mukhopadhyay, M. (2018). Use of Fermentation Technology for Value-Added Industrial Research. Principles and Applications of Fermentation Technology, August, 141–161. https://doi.org/10.1002/9781119460381.ch8
- Hind, H. L., & Day, F. E. (1930). Fermentation Industries. In Journal of the Institute of Brewing (Vol. 36, Issue 6, pp. 1–29). https://doi.org/10.1002/j.2050-0416.1930.tb05286.x
- Landine, R., De Garie, C., & Cocci, A. (1997). Fermentation process. Biotechnology Advances, 15(3–4), 702. https://doi.org/10.1016/s0734-9750(97)87650-1
- Martínez-Espinosa, R. M. (2020). Introductory Chapter: A Brief Overview on Fermentation and Challenges for the Next Future. New Advances on Fermentation Processes. https://doi.org/10.5772/INTECHOPEN.89418
- Microbiology, F. (2016). Basic Principles of Food Fermentation. Food Microbiology: Principles into Practice, 228–252. https://doi.org/10.1002/9781119237860.ch39
- Principles and Applications of Fermentation Technology. (2018). In Principles and Applications of Fermentation Technology. https://doi.org/10.1002/9781119460381
- Principles of Fermentation Technology Second Edition. (n.d.).
- Sharma, R., Garg, P., Kumar, P., Bhatia, S. K., & Kulshrestha, S. (2020). Microbial fermentation and its role in quality improvement of fermented foods. Fermentation, 6(4), 1–20. https://doi.org/10.3390/fermentation6040106.
