Alcoholic beverages include ethanol, sometimes referred to as ethyl alcohol, a psychoactive chemical. When ingested, ethanol goes through a long metabolic process in the body. Understanding how ethanol is metabolized is essential to understanding how it affects human behavior and health. The physiology of ethanol metabolism is examined in this article, along with organic reaction plans, thermodynamic factors, and the function of genes in this procedure.
Interesting Science Videos
Ethanol Metabolism Location
Cells of the kidney and liver. Greater than 80% of the absorbed ethanol is metabolized in the liver.
Human Metabolic Physiology
- Ethanol is metabolized in the human body through a number of metabolic and physiological processes.
- Alcohol dehydrogenase (ADH) is the main enzyme involved in turning ethanol into acetaldehyde.
- Acetaldehyde is toxic and is further metabolized into acetic acid by acetaldehyde dehydrogenase (ALDH).
- Acetic acid coenzyme A ligase (ACSL) breaks down acetic acid into acetyl-CoA, which can participate in the Krebs cycle or citric acid cycle.
- Ethanol metabolism is the breakdown of acetyl-CoA in the mitochondria, producing water and carbon dioxide, and energy in the form of ATP.
Individual differences, gender, body composition, and liver disease can all have an impact on ethanol metabolism. Chronic and excessive alcohol consumption can lead to liver damage. Other organs such as the brain and gastrointestinal tract also play a role in ethanol metabolism.
Ethanol and Evolution
- The ability to metabolize ethanol is not unique to humans; it has evolved in various organisms over millions of years.
- Ethanol is a product of fermentation, a metabolic pathway that has existed for billions of years.
- Humans, like other mammals, possess enzymes that allow them to break down ethanol.
Physiologic Structures
- Ethanol metabolism primarily occurs in the liver, where hepatocytes play a vital role.
- Hepatocytes contain specific enzymes responsible for the oxidation of ethanol and its conversion into less toxic metabolites.
Thermodynamic Considerations
- Ethanol metabolism involves several thermodynamic considerations, which relate to the energy changes that occur during the breakdown of ethanol and its subsequent conversion into metabolites.
- These thermodynamic processes are essential for understanding the energetics of ethanol metabolism.
Energy Thermodynamics
Multiple thermodynamic factors are taken into account during the metabolism of ethanol. The entire process of ethanol metabolism results in the release of energy, which is calculable using thermodynamics. Alcohol metabolism is an exothermic process, which means that energy is released. The general ethanol metabolic response is as follows:
Ethanol + NAD+ + H2O → Acetaldehyde + NADH + H+ + 2e^-
This reaction involves the oxidation of ethanol and the reduction of NAD+ to NADH. The released energy is harnessed in the form of high-energy electrons, which are transferred to the electron carrier NADH.
Energy Calculations
The metabolism of ethanol may be broken down into a number of processes, each having distinct thermodynamic characteristics.
- In step one, ethanol is transformed into acetaldehyde, in step two, acetaldehyde is transformed into acetic acid, and in step three, acetic acid is transformed into acetyl-CoA.
- Acetyl-CoA is broken down into water and CO2 in steps four through eleven. The net energy released during ethanol metabolism is determined by doing energy calculations for each step.
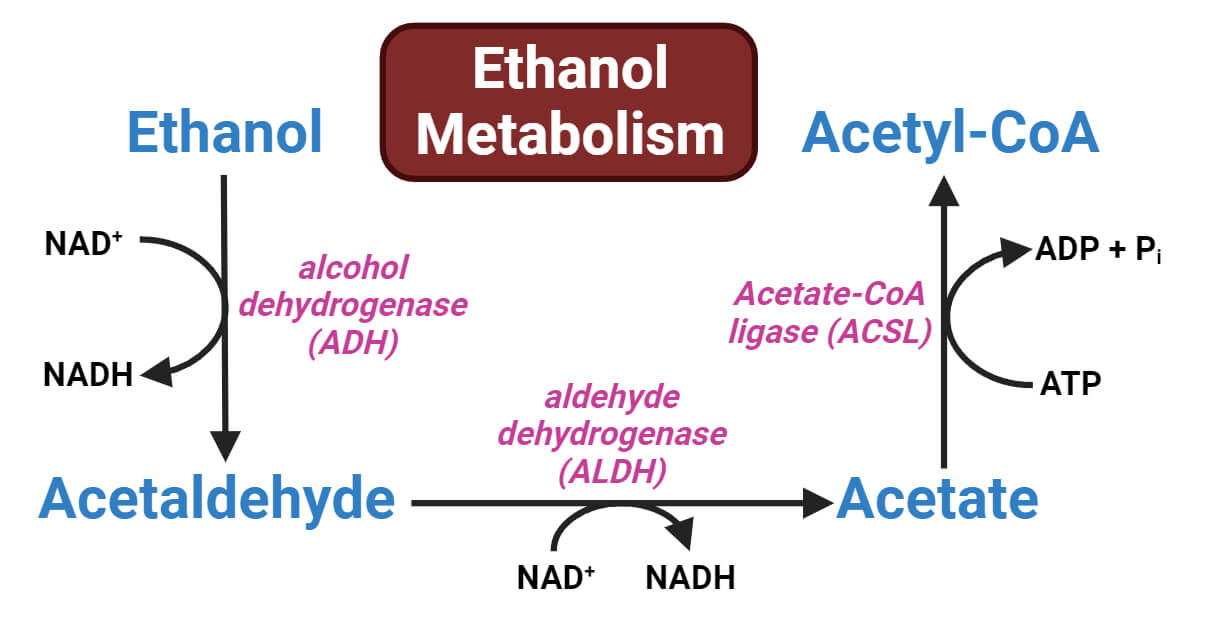
Ethanol Metabolism Steps
The process of ethanol metabolism can be divided into several steps, each with its own thermodynamic properties. Let’s examine the major steps involved:
Step One: Ethanol to Acetaldehyde
- The first step involves the conversion of ethanol to acetaldehyde by the enzyme alcohol dehydrogenase (ADH).
- This reaction releases energy in the form of heat.
- The energy released can be calculated by considering the change in the enthalpy (∆H) of the reaction.
Step Two: Acetaldehyde to Acetic Acid
- Acetaldehyde is further metabolized to acetic acid by the enzyme acetaldehyde dehydrogenase (ALDH).
- The conversion of acetaldehyde to acetic acid is an exothermic reaction, releasing energy.
- The change in enthalpy (∆H) of this reaction can also be calculated.
Step Three: Acetic Acid to Acetyl-CoA
- The enzyme acetic acid coenzyme A ligase (ACSL) catalyzes the conversion of acetic acid to acetyl-CoA.
- This step involves the formation of a high-energy thioester bond between acetic acid and coenzyme A (CoA).
- The hydrolysis of this bond releases energy, which can be quantified by considering the change in enthalpy (∆H) of the reaction.
Steps 4 through 11: Acetyl-CoA Breakdown
- The subsequent steps involve the breakdown of acetyl-CoA through the citric acid cycle and oxidative phosphorylation, resulting in the production of water and carbon dioxide.
- These processes are highly exergonic, meaning they release significant amounts of energy.
Discussion of Calculations
- Analyzing the energy calculations for each step provides insights into the overall energy balance of ethanol metabolism.
- The net energy released during the breakdown of ethanol contributes to the body’s energy supply.
- However, it is important to note that the efficiency of energy production from ethanol metabolism is relatively low compared to other energy sources, such as glucose or fatty acids.
- The body can use the energy generated during the metabolism of ethanol for ATP production and other metabolic activities.
- On the other hand, excessive alcohol use can cause an imbalance in energy metabolism and support the emergence of illnesses linked to alcohol, such as liver damage.
- It is important to note that the intricacy of ethanol metabolism cannot be fully understood by thermodynamic considerations alone.
- The total process is also influenced by other variables, including substrate availability, regulatory mechanisms, and enzyme kinetics.
Organic Reaction Scheme
The organic reaction scheme in ethanol metabolism involves the conversion of ethanol to acetaldehyde, acetaldehyde to acetic acid, acetic acid to acetyl-CoA, and the subsequent breakdown of acetyl-CoA in the citric acid cycle.
Ethanol Metabolism Reaction
- Ethanol metabolism adheres to a clearly laid out organic reaction pattern.
- The oxidation of ethanol to acetaldehyde is catalyzed in the first step by the enzyme alcohol dehydrogenase (ADH).
- The second step is made easier by the enzyme aldehyde dehydrogenase (ALDH), which turns acetaldehyde into acetic acid.
- In the third step, acetic acid is converted to acetyl-CoA by the enzyme acetic acid coenzyme A ligase (ACSL).
- In the mitochondria, acetyl-CoA is further metabolized to create water and carbon dioxide in stages four through eleven.
Gene Expression and Ethanol Metabolism
The regulation of ethanol metabolism in people is greatly influenced by gene expression. The efficiency of ethanol metabolism is determined by the expression of particular genes and the activity of the relevant enzymes, which also affects an individual’s tolerance to alcohol and susceptibility to alcohol-related health issues. Here, we’ll look at the patterns of gene expression and how they affect ethanol metabolism at various phases of the process.
Ethanol to Acetaldehyde
- Ethanol is converted to acetaldehyde through the action of the enzyme alcohol dehydrogenase (ADH).
- This reaction involves the oxidation of ethanol and the transfer of hydride ions (H-) to the coenzyme NAD+.
- The reaction can be represented as follows: Ethanol + NAD+ → Acetaldehyde + NADH + H+
Ethanol to Acetaldehyde in Human Adults
- The conversion of ethanol to acetaldehyde in human adults is mainly mediated by the enzyme alcohol dehydrogenase (ADH).
- Different genetic variants of ADH exist in the human population, resulting in variations in ethanol metabolism and alcohol tolerance.
Ethanol to Acetaldehyde in Human Fetuses
- The metabolism of ethanol in human fetuses differs from that in adults.
- Fetal alcohol dehydrogenase (FADH) is the primary enzyme responsible for the conversion of ethanol to acetaldehyde.
- However, fetal ADH activity is relatively low compared to adults, leading to higher acetaldehyde levels in the fetus.
Acetaldehyde to Acetic Acid
- The conversion of acetaldehyde to acetic acid is catalyzed by the enzyme acetaldehyde dehydrogenase (ALDH).
- ALDH plays a crucial role in reducing acetaldehyde levels, as it is a toxic substance and contributes to alcohol-induced tissue damage.
- This reaction is catalyzed by the enzyme acetaldehyde dehydrogenase (ALDH). Acetaldehyde is oxidized, and NAD+ is reduced to NADH in the process.
- The reaction can be represented as: Acetaldehyde + NAD+ + H2O → Acetic Acid + NADH + H+
Acetic Acid to Acetyl-CoA
- The enzyme acetic acid coenzyme A ligase (ACSL) facilitates the conversion of acetic acid to acetyl-CoA.
- Acetyl-CoA is an important metabolite in various metabolic pathways, including the citric acid cycle and fatty acid synthesis.
- First, acetic acid reacts with coenzyme A (CoA) in the presence of the enzyme acetic acid coenzyme A ligase (ACSL) to form acetyl-CoA.
- The reaction is as follows: Acetic Acid + CoA + ATP → Acetyl-CoA + AMP + PPi
Acetyl-CoA breakdown
- The citric acid cycle, commonly referred to as the Krebs cycle or the TCA cycle, is where acetyl-CoA is produced by the metabolism of ethanol enters the mitochondria.
- Acetyl-CoA undergoes oxidative decarboxylation, which results in the production of ATP, NADH, and FADH2 as well as energy.
- In the end, the citric acid cycle completely oxidizes acetyl-CoA to carbon dioxide and regenerates the initial molecule, oxaloacetate.
- The breakdown of acetyl-CoA in the mitochondria is one of the last processes of ethanol metabolism.
- Water and carbon dioxide are produced as a result of further metabolizing of acetyl-CoA through a sequence of processes.
- Through oxidative phosphorylation, this process produces energy in the form of adenosine triphosphate (ATP).
In general, ethanol is metabolized by first becoming acetaldehyde, which is then transformed into acetic acid. Acetyl-CoA is then produced from acetic acid and enters the citric acid cycle to produce further energy. Acetyl-CoA is completely broken down, releasing carbon dioxide, water, and ATP as well as energy. It’s critical to remember that these processes are mediated by particular enzymes and are influenced by a variety of variables, such as genetic differences, hormone signals, and metabolic status. Additionally, characteristics including age, gender, and liver function might have an impact on how each person processes ethanol.
Ethanol Metabolism Related Diseases
- Acetaldehyde is an unstable molecule, which is prone to forming free radicals, which can be toxic to the liver (leading to cirrhosis).
- Acetaldehyde can also be damaging to embryological neural crest tissue and is thought to be involved in the neurologic manifestations of fetal alcohol syndrome.
- Alcoholics are at risk for hypoglycemia when they ingest ethanol.
- The increased ratio of NADH/NAD+, which results from the metabolism of ethanol, causes pyruvate and oxaloacetate to be reduced to lactate and malate, respectively.
- Because pyruvate and oxaloacetate are intermediates in gluconeogenesis, gluconeogenesis is impaired and hypoglycemia can result.
Conclusion
A number of enzymatic processes and metabolic pathways are involved in the complicated physiological process of ethanol metabolism. As ethanol metabolism has developed over time, it is now possible for humans and other living things to digest and remove this intoxicating molecule.
Understanding the chemical reaction scheme, the function of gene expression, and the thermodynamic factors involved in ethanol metabolism will help you better understand how it affects the body. The ability to tolerate alcohol and the likelihood of developing alcohol-related health issues are both significantly influenced by ethanol metabolism.
The way a person reacts to alcohol, including the likelihood of developing alcohol-related liver illness, can be affected by variations in the genes encoding important enzymes involved in the metabolism of ethanol.
References
- Zakhari, Samir. “Overview: how is alcohol metabolized by the body?.” Alcohol research & health 29.4 (2006): 245.
- Ethanol Metabolism – https://www.lecturio.com/concepts/ethanol-metabolism/
- Ethanol Metabolism – https://www.sciencedirect.com/topics/neuroscience/ethanol-metabolism
- Crabb, David W., William F. Bosron, and T-K. Li. “Ethanol metabolism.” Pharmacology & therapeutics 34.1 (1987): 59-73.
- Wilson, David F., and Franz M. Matschinsky. “Ethanol metabolism: The good, the bad, and the ugly.” Medical hypotheses 140 (2020): 109638.
- Alexandre, H., et al. “Global gene expression during short-term ethanol stress in Saccharomyces cerevisiae.” FEBS letters 498.1 (2001): 98-103.
- Roden, Eric E., and Qusheng Jin. “Thermodynamics of microbial growth coupled to metabolism of glucose, ethanol, short-chain organic acids, and hydrogen.” Applied and environmental microbiology 77.5 (2011): 1907-1909.
- David Hames and Nigel Hooper (2005). Biochemistry. Third ed. Taylor & Francis Group: New York.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- https://pubs.niaaa.nih.gov/publications/aa72/aa72.htm
