Western blot, also known as immunoblotting, is the process of separating proteins and identifying them in a complex biological sample.
- The use of polyacrylamide gel electrophoresis is a prerequisite for western blotting in order to separate proteins prior to their identification.
- The process of western blotting involves the transfer of proteins separated by SDS PAGE into an absorbent membrane. The proteins can then be identified on the membrane by different means.
- Western blotting has revolutionized the field of immunology with the use of antibody probes against membrane-bound proteins.
- The immunodetection of proteins has a wide application in biochemistry and other sciences as it can detect and characterize a multitude of proteins.
- The sensitivity of the process depends on the efficiency of transfer retention of proteins during processing and the final detection.
- Western blotting or protein blotting depends on the specificity of interaction between the protein of interest and the probe used for the detection of the protein.
- Unlike Southern blotting that utilizes radio-labeled nucleic acid probes, western blotting usually uses a second antibody tagged with an enzyme.
- Western blotting has a number of advantages over other similar techniques as the process only requires the use of a small amount of reagents, and the same protein transfer can be used for multiple analyses.
Interesting Science Videos
Principle of Western Blot
- The principle of western blotting is the interaction between the proteins and the probes used for the detection of the proteins.
- The proteins used for western blotting are separated by gel electrophoresis to obtain them on a gel matrix.
- The proteins are then transferred to a nitrocellulose or polyvinylidene fluoride (PVDF) membrane, where they are immobilized. The transfer of the protein is known as blotting.
- The protein on the membrane can either be detected by the use of a reporter-labeled primary antibody directed against the protein or a reporter-labeled secondary antibody directed at the primary antibody.
- The reporter or probe present on the antibody can be an enzyme that produces a color reaction or a luminescent signal at the antigen-antibody binding site that produces a fluorescent signal in the presence of a particular substrate.
- The signal or color generated by the probe requires a detection system that is appropriate for the signal or intensity generated.
Requirements for Western Blot
Gel Electrophoresis
- Gel (NuPAGE)
- Basic Power Supply
- Sample buffer
- Heating block
- Sample reducing buffer
- Pre-stained protein ladder
Protein Transfer
- Mini Trans-Blot consisting of a tank, lid, and a blot module. The assembly is stored with cooling ice so that it is frozen when needed.
- 0.45 µm nitrocellulose filter paper
- NuPAGE transfer buffer
- Methanol
- Square Pyrex dish
- Square disposable plastic Petri dish
- Razorblade and gel knife
- Basic power supply
Western Blotting
- 10x Tris-buffered saline with 1% Tween 20.
- Shaker
- 10% powdered nonfat dry milk
- Square disposable plastic Petri dish
- Plastic pouches
- Impulse heat sealer
- Primary antibody
- Secondary antibody conjugated with horseradish peroxidase against the host species of the primary antibody.
- Chemiluminescent substrate
- Gel documentation system
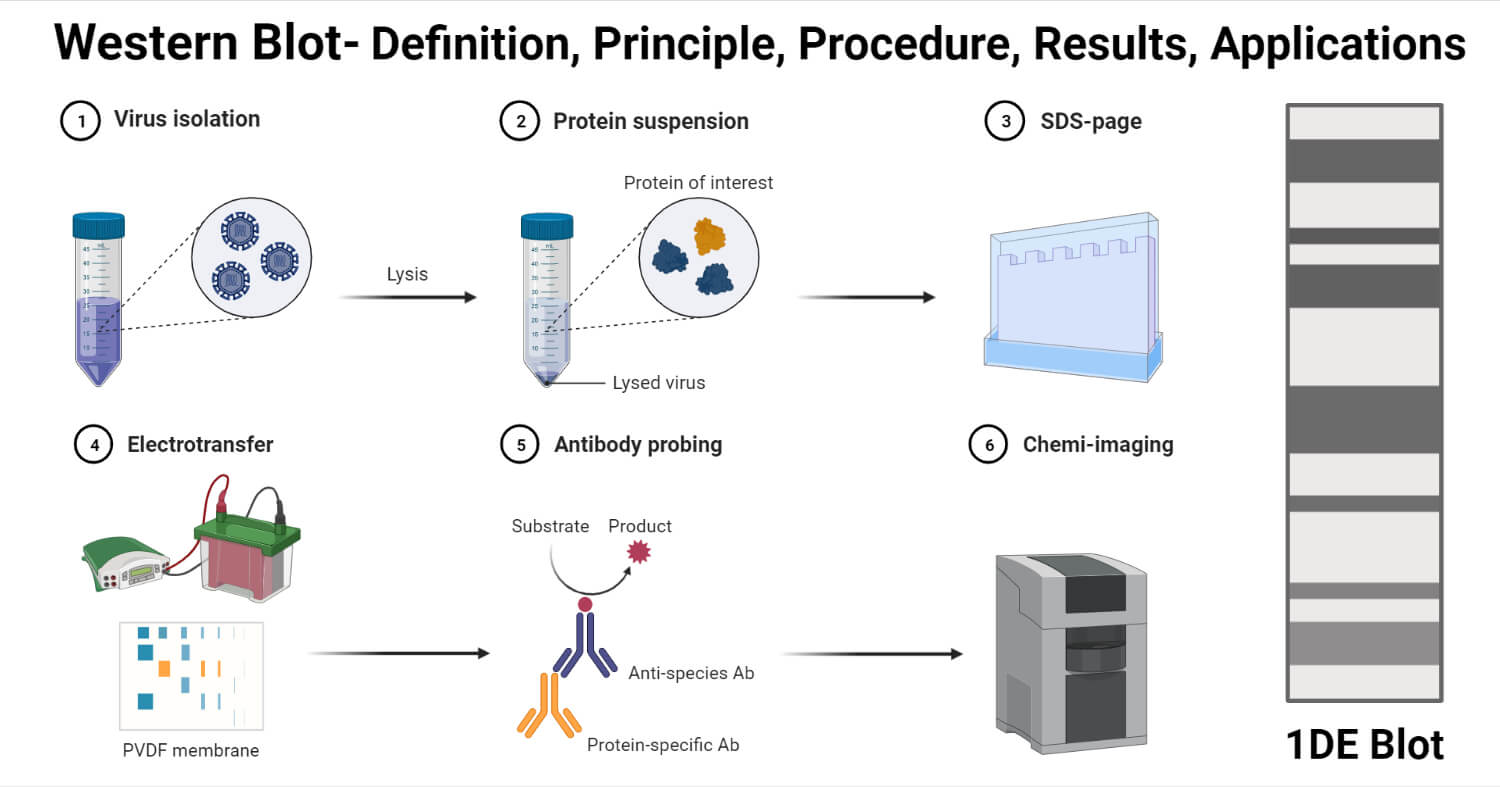
Procedure of Western Blot
The process of western blotting consists of the following steps;
1. Sample Preparation
- The most commonly used samples for western blot are cell lysates which are collected by the process of extraction.
- The extraction can be achieved by different means like mechanical destruction, chemical extraction, or the use of enzymes.
- The extraction of often performed at cold temperature in the presence of protease inhibitors in order to prevent the denaturation of the proteins.
2. Gel Electrophoresis
- The protein sample is diluted with the sample buffer and is heated and shaken for 10 minutes at 70°C.
- The sample is then centrifuged at 5000g.
- The gel case is removed from the pouch and is placed in the buffer tank against the rubber seal with the gel walls facing the inside of the tank reservoir.
- The running buffer is poured onto the upper reservoir while ensuring that no buffer leakage occurs on the lower tank.
- Each of the wells is then loaded with an equal volume of heat-denatured sample, and one of the lanes is reserved for the protein ladder.
- The lid is placed on the tank, and it is connected to the power supply.
- The run is allowed to run at 200 V constant for 50 minutes.
3. Protein Transfer
- The transfer buffer is prepared by adding 10% methanol to the buffer.
- The transfer case is taken and laid out. It is then covered with a transfer buffer.
- A foam sponge is taken and laid on the backside, over which goes the filter paper. These should be placed to ensure that both of them are wet and slightly submerged.
- The gel is taken out from the tank and placed on the wet filter paper.
- The nitrocellulose membrane is wet with the transfer buffer and is placed on top of the gel in a way that there are no bubbles between the gel and the membrane.
- The transfer case is placed into the transfer tank, which is further filled with transfer buffer.
- The tank is then connected to power at 100V for 1 hour.
- Once the transfer is complete, the transfer case is removed, and the nitrocellulose membrane is removed from the gel.
4. Immunodetection
- The membrane is washed with Tris-buffered saline for 5 minutes in a Petri dish.
- The 10% nonfat dry milk is mixed with the Tris buffer, and the membrane is covered with the mixture for 30 minutes at room temperature.
- The membrane is washed with the Tris buffer to remove any excess mixture remaining on the membrane.
- With the help of the forceps, the membrane is transferred to a new Petri dish onto which the primary antibody is added.
- The membrane with the antibody is incubated for 3 hours at room temperature. The membrane is washed after incubation with the Tris buffer.
- The membrane is transferred again to a new Petri dish, where a secondary HRP-conjugated antibody is added. The membrane is incubated for 1 hour. The concentration of secondary antibodies often remains at 1 µg/ml, but this also depends on the dilution.
- The membrane is washed again with Tris buffer to remove excess antibodies from the surface.
- The membrane is incubated with the substrate for 5 minutes, and the observation is made.
Result Interpretation of Western Blot
- The result of western blotting depends on the type of probes used during the process.
- If an enzyme-conjugated secondary antibody is used, the reaction between the substrate and the enzyme produces a color.
- The soluble dye is converted into an insoluble form, resulting in a different color on the membrane.
- In order to stop the development of a blot, the dye is removed by washing the membrane.
- The protein levels can then be evaluated by spectrophotometry.
Applications of Western Blot
- Western blotting is an excellent method with high sensitivity in order to detect a particular protein even in low quantity.
- Western blotting has been used in the clinical diagnosis of different diseases. The confirmatory test for HIV involves a western blot by detecting anti-HIV antibodies in the serum.
- The technique has been used to quantify proteins and other gene products in gene expression studies.
- Since western blotting detects the proteins by their size and ability to bind to the antibody, it is appropriate for evaluating the protein expressions in cells and further analysis of protein fractions during protein purification.
- Western blotting is also used for the analysis of different biomarkers like growth factors, cytokines, and hormones.
Limitations of Western Blot
- Since it is a very sensitive process, any imbalance in the process can affect the results of the entire process.
- In some cases, no bands or erroneous bands might be observed due to the insufficient transfer of the proteins.
- The test can only be used as a semi-quantitative test as the estimation is not always precise.
- The process is time-consuming and complex, thus can only be performed by well-trained personnel.
- Western blotting can only be performed for proteins if the primary antibodies for the proteins are available.
- Some antibodies might exhibit off-target effects by interacting with more than one protein in the sample.
- The technique is a costly process with the cost of antibodies and expensive detection methods.
- Small proteins might not be retained by the membrane, whereas larger proteins are difficult to transfer to the membrane.
References
- Ghosh, Rajeshwary et al. “The necessity of and strategies for improving confidence in the accuracy of western blots.” Expert review of proteomics vol. 11,5 (2014): 549-60. doi:10.1586/14789450.2014.939635
- Mahmood, Tahrin, and Ping-Chang Yang. “Western blot: technique, theory, and trouble shooting.” North American journal of medical sciences vol. 4,9 (2012): 429-34. doi:10.4103/1947-2714.100998
- Hnasko TS, Hnasko RM. The Western Blot. Methods Mol Biol. 2015;1318:87-96. doi: 10.1007/978-1-4939-2742-5_9. PMID: 26160567.
- Kurien BT, Scofield RH. Western blotting: an introduction. Methods Mol Biol. 2015;1312:17-30. doi: 10.1007/978-1-4939-2694-7_5. PMID: 26043986; PMCID: PMC7304528.
Sources
- https://www.cytivalifesciences.com/en/us/solutions/protein-research/knowledge-center/western-blotting/protein-immunoblotting-overview – 9%
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4791038/ – 8%
- https://www.thermofisher.com/us/en/home/references/ambion-tech-support/northern-analysis/tech-notes/membrane-transfer-and-crosslinking-for-rna.html – 1%
- https://www.thermofisher.com/de/de/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-electrophoresis.html – 1%
- http://www.fao.org/3/Y5013E/y5013e07.htm – 1%
- https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_2A%3A_Introductory_Biology_(Easlon)/Readings/15.3%3A_Membrane_Transport_with_Selective_Permeability – 1%
- https://www.onlinebiologynotes.com/western-blotting-technique-principle-procedure-application/ – 1%
- https://www.sciencedirect.com/science/article/pii/S1046202306000065 – 1%
- https://www.academia.edu/19889617/Protein_blotting_a_review – 1%
- https://en.wikipedia.org/wiki/Gel_extraction – 1%
- https://www.700r4transmissionhq.com/symptoms-of-transfer-case-problems/ – 1%
- http://ww2.justanswer.com/uploads/mcvgreg/2008-07-04_212231_2002-TrailBlazer-TC-Desc-Operation.pdf – <1%

Great work