The key metabolic system known as the urea cycle is essential for the body’s process of excreting extra nitrogen. For the creation of proteins, nucleic acids, and other important compounds, nitrogen is a crucial component. However, the buildup of poisonous ammonia brought on by the breakdown of proteins may be detrimental to cells and tissues. The urea cycle offers a method for turning ammonia into urea, a less harmful substance that the kidneys may safely eliminate. The body’s process of excreting excess nitrogen depends on the fundamental metabolic pathway known as the urea cycle.
- Nitrogen is an essential element for synthesizing proteins, nucleic acids, and other vital molecules.
- However, cells and tissues may suffer from the accumulation of toxic ammonia caused by the breakdown of proteins.
- Ammonia may be converted into urea, a less dangerous chemical that the kidneys can safely remove via the urea cycle.
Interesting Science Videos
Nitrogen and Mitochondrial Matrix
- In the mitochondrial matrix, citrulline is created when ornithine and carbamoyl phosphate are combined.
- The cytoplasm is where further reactions happen once citrulline has been delivered there.
- Aspartate is incorporated, argininosuccinate is broken down, and arginine is hydrolyzed during these processes, creating urea.
- It is essential to control the urea cycle in order to preserve nitrogen homeostasis.
- The urea cycle enzymes’ activity is influenced by a number of variables, such as substrate concentrations, allosteric activators, and interactions with other metabolic processes.
Urea cycle diseases, a class of hereditary metabolic abnormalities characterized by deficits in certain urea cycle enzymes, can result from disruptions in this control. For these illnesses to be correctly diagnosed and treated, it is crucial to comprehend the urea cycle and the ailments that it is linked to. Additionally, it sheds light on wider facets of nitrogen metabolism and how closely linked the body’s metabolic processes are. We get a greater comprehension of the underlying mechanisms that control cellular metabolism and contribute to the general health of the human body by digging into the fine details of the urea cycle.
Urea Cycle Functions
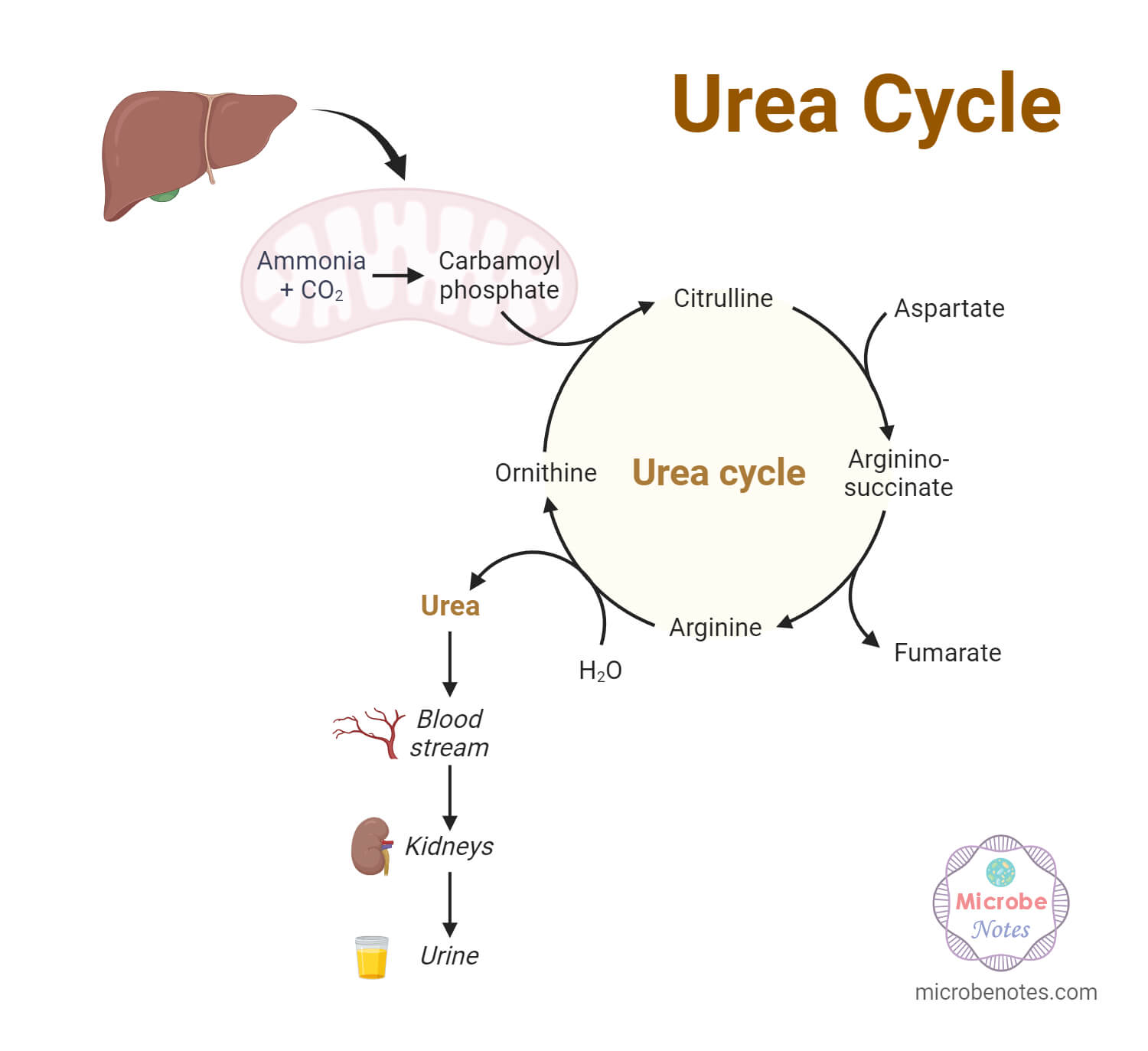
The urea cycle is a crucial metabolic pathway responsible for eliminating excess nitrogen from the body. Nitrogen is obtained through dietary proteins and other nitrogen-containing compounds, and its removal is essential to prevent toxic buildup. The urea cycle takes place primarily in the liver, where it converts toxic ammonia, derived from protein breakdown, into less toxic urea, which can be safely excreted by the kidneys.
Urea Cycle Reactions
The urea cycle consists of a series of enzymatic reactions that take place in the mitochondria and cytoplasm of hepatocytes (liver cells). Let us delve into the key reactions involved in this process.
First Reaction: Entering the Urea Cycle
- The initial step of the urea cycle involves the conversion of toxic ammonia (NH3) to a less toxic compound called carbamoyl phosphate.
- This reaction is catalyzed by the enzyme carbamoyl phosphate synthetase I (CPS I), which requires two ATP molecules and bicarbonate (HCO3-) as substrates.
- CPS I is primarily located in the mitochondria of hepatocytes.
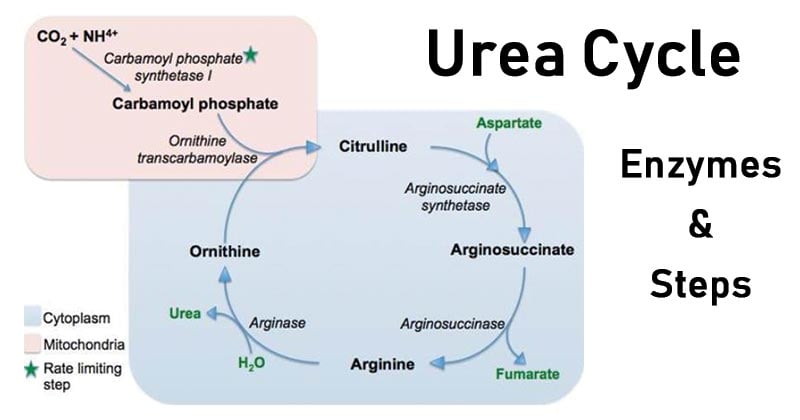
Second Reaction – Urea Cycle in Mitochondria
- In the mitochondrial matrix, carbamoyl phosphate is created and then reacts with ornithine to create citrulline.
- Ornithine transcarbamylase is an enzyme that promotes this process.
- The remaining processes of the urea cycle subsequently proceed in the cytoplasm once citrulline has been transferred over the inner mitochondrial membrane.
- Argininosuccinate synthetase catalyzes the process in the cytoplasm where citrulline and aspartate combine to generate argininosuccinate.
- Argininosuccinate lyase further cleaves argininosuccinate to provide fumarate and arginine.
- After being hydrolyzed by arginase, arginine produces urea and ornithine, both of which can re-enter the mitochondrial matrix and take part in the urea cycle once more.
Overall Reaction Equation
The overall reaction equation for the urea cycle is as follows:
Ammonia + Aspartate + 3 ATP + 2 HCO3- → Urea + Fumarate + 2 ADP + AMP + 2 Pi + PPi + 2 H2O
Essential enzymes in the Urea Cycle
- Carbamoyl phosphate synthetase I: Converts ammonium and bicarbonate into carbamoyl phosphate. This is the rate-limiting step in the urea cycle. This reaction requires two ATP and occurs in the mitochondria.
- Ornithine transcarbamoylase: Combines ornithine and carbamoyl phosphate to form citrulline. Located in mitochondria.
- Argininosuccinate synthetase: Condenses citrulline with aspartate to form arginosuccinate. This reaction occurs in the cytosol and requires one ATP.
- Argininosuccinate lyase: Splits argininosuccinate into arginine and fumarate. Occurs in the cytosol.
- Arginase: Cleaves arginine into one molecule of urea and ornithine in the cytosol. The ornithine is then transported back into the mitochondria for entry back into the cycle.
Products of the Urea Cycle
In the urea cycle, poisonous ammonia is changed into urea, a less dangerous substance that may be safely eliminated from the body. The urea cycle’s main output is urea itself. However, during the various stages of the urea cycle, a number of other chemicals are also created as intermediate products.
The overall reaction equation of the urea cycle indicates the production of several products:
Ammonia + Aspartate + 3 ATP + 2 HCO3- → Urea + Fumarate + 2 ADP + AMP + 2 Pi + PPi + 2 H2O
1. Urea
The main byproduct of the urea cycle is urea. It is a nitrogenous waste product created by a sequence of enzymatic interactions between ammonia and carbon dioxide. Water-soluble urea is carried to the kidneys where it is excreted through urine.
2. Fumarate
An intermediary substance created during the urea cycle is fumarate. It is produced when the enzyme argininosuccinate lyase cleaves argininosuccinate, an intermediate in the urea cycle. Fumarate can engage in cellular respiration and the Krebs cycle, which are both processes that help cells produce energy.
3. ADP and AMP
Adenosine triphosphate (ATP) is hydrolyzed during the urea cycle to create the nucleotides ADP (adenosine diphosphate) and AMP (adenosine monophosphate). Numerous cellular functions employ ATP as an energy source, and its breakdown into ADP and AMP releases energy needed for the enzymatic enzymes involved in the urea cycle.
4. Pi (Inorganic Phosphate) and PPi (Pyrophosphate)
Inorganic phosphate (Pi) and pyrophosphate (PPi) are also byproducts of ATP hydrolysis during the urea cycle. These molecules play important roles in cellular metabolism and serve as phosphate donors or acceptors in various biochemical reactions.
5. Water (H2O)
Water is a product of the hydrolysis reactions that occur during the urea cycle. These hydrolysis reactions involve the breakdown of ATP and the cleavage of argininosuccinate, resulting in the release of water molecules.
The complex metabolic processes required in converting harmful ammonia into the less toxic and more readily excreted urea are reflected in the creation of these different molecules during the urea cycle. Our grasp of nitrogen metabolism and the interconnection of metabolic pathways inside the body is aided by our understanding of the urea cycle’s byproducts.
Urea Cycle Regulation
The urea cycle is tightly regulated to maintain proper nitrogen balance in the body. Several mechanisms are involved in the regulation of this metabolic pathway.
N-Acetylglutamic Acid
- As an allosteric activator of CPS I, the enzyme that catalyzes the first step in the urea cycle, N-Acetylglutamic acid (NAG) is used.
- NAG levels that are higher encourage CPS I activity, which improves how well the urea cycle removes extra ammonia.
Substrate Concentrations
- The urea cycle is regulated in part by the availability of substrates.
- While large quantities of citrulline, arginine, or urea help avoid excessive urea formation, high concentrations of ammonia and ornithine promote the advancement of the urea cycle.
Link with the Citric Acid Cycle
- The urea cycle is intimately linked with the citric acid cycle (also known as the Krebs cycle) in cellular metabolism.
- The citric acid cycle provides the precursor molecule, fumarate, which is generated during the urea cycle.
- Fumarate is produced when argininosuccinate is cleaved, and it can re-enter the citric acid cycle as an intermediate, contributing to the overall energy production and metabolic integration.
Urea Cycle Disorders (UCD)
A category of hereditary metabolic illnesses known as urea cycle disorders (UCDs) affects how well the urea cycle’s enzymes work. Hyperammonemia is brought on by several conditions, which cause poisonous ammonia to build up in the body. Deficiencies in CPS I, ornithine transcarbamylase, argininosuccinate synthetase, argininosuccinate lyase, or arginase are only a few of the possible manifestations of UCDs. The symptoms might be minor or potentially fatal, necessitating quick medical attention.
Individual Disorders
Each urea cycle disorder is characterized by a specific enzyme deficiency:
1. Carbamoyl phosphate synthetase I (CPS I) deficiency
2. Ornithine transcarbamylase (OTC) deficiency
3. Citrullinemia (argininosuccinate synthetase deficiency)
4. Argininosuccinic aciduria (argininosuccinate lyase deficiency)
5. Argininemia (arginase deficiency)
Symptoms and Treatments
- Lethargy, poor eating, vomiting, seizures, and developmental abnormalities are among the signs of these illnesses that frequently manifest.
- Combining dietary changes, medicine, and, in extreme circumstances, liver transplantation, is the course of treatment.
- Clinical symptoms, blood tests to assess ammonia and amino acid levels, and genetic testing to identify particular enzyme deficits are frequently used to diagnose urea cycle diseases.
- To stop hyperammonemia-related life-threatening consequences, early identification and management are essential.
- Restoring the equilibrium of nitrogen metabolism and reducing ammonia accumulation in the body are the goals of treatment for urea cycle diseases.
- This frequently entails following a low-protein diet, which limits the consumption of substances that contain nitrogen, such as proteins and certain amino acids.
- Additionally, certain drugs may be administered to improve ammonia excretion or offer other routes for its clearance.
- Since the liver is the main location for the generation of urea cycle enzymes, liver transplantation may be an option in extreme situations.
A multidisciplinary strategy combining medical experts, such as metabolic specialists, dietitians, and genetic counselors is necessary to treat urea cycle diseases. To avoid acute bouts of hyperammonemia and to guarantee ideal growth and development, regular monitoring of ammonia levels and nutritional status is necessary. Ongoing studies are being conducted to better comprehend the underlying causes of urea cycle diseases and to create fresh approaches to treating them. Emerging research areas including gene therapy and enzyme replacement therapy provide promise for improvements in the treatment of these ailments in the future.
Conclusion
In conclusion, the urea cycle is a fundamental metabolic pathway that plays a vital role in maintaining nitrogen balance within the body. By converting toxic ammonia into urea, the urea cycle enables the safe excretion of excess nitrogen, preventing its harmful accumulation. The series of reactions and regulations involved in the urea cycle ensure its efficient functioning and coordination with other metabolic pathways.
Disorders of the urea cycle, though rare, can have severe consequences due to the impaired enzymatic activity, leading to hyperammonemia and potential neurological damage. Early diagnosis, appropriate management, and ongoing research efforts are crucial in addressing these disorders and improving patient outcomes.
The study of the urea cycle and its associated disorders not only expands our understanding of nitrogen metabolism but also provides valuable insights into broader aspects of human biochemistry and genetic disorders.
By exploring the complexities of the urea cycle, we continue to unravel the intricate mechanisms underlying human physiology, paving the way for advancements in diagnostic techniques, therapeutic interventions, and potential gene-based treatments for urea cycle disorders.
References
- Physiology, Urea Cycle – https://www.ncbi.nlm.nih.gov/books/NBK513323/
- Steps of the Urea Cycle – https://byjus.com/biology/steps-of-the-urea-cycle/
- Urea Cycle Disorder – https://my.clevelandclinic.org/health/diseases/23470-urea-cycle-disorder
- Hereditary urea cycle abnormality – https://medlineplus.gov/ency/article/000372.htm
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- Madigan, M. T., Martinko, J. M., Bender, K. S., Buckley, D. H., & Stahl, D. A. (2015). Brock biology of microorganisms (Fourteenth edition.). Boston: Pearson.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
