
Interesting Science Videos
Benedict’s test for reducing sugars
Definition
Benedict’s test is a biochemical test performed to distinguish reducing sugars (monosaccharides and some disaccharides) from non-reducing sugars.
Objectives
- To detect the presence of simple carbohydrates in a solution.
- To distinguish between reducing and non-reducing sugars.
Principle
- The carbohydrates having a free or potentially free, aldehyde or ketone group can act as a reducing agent.
- In order to detect the reducing agent, Benedict’s reagent is used. It appears deep blue in color and consists of copper sulfate mixed with sodium citrate and a weak alkali, sodium carbonate.
- When reducing sugars are heated in the presence of alkali, they get converted to enediols, which are powerful reducing agents.
- Enediols reduce the cupric ions (Cu2+) present in the benedict’s reagent to cuprous ions (Cu+), which get precipitated as insoluble red-colored cuprous oxide.
- The test is semi-quantitative since the color of the precipitate indicates an approximate quantity of the sugar present in the sample.
- For a sample containing reducing sugar the color of the sample during boiling progress from blue (with no reducing sugar present), green, yellow, orange, red, and then brick red or brown (with a high concentration of reducing sugars).
- The citrate ions form a complex with cupric ions and prevent its precipitation with the hydroxide ions as cupric hydroxide.
Reaction
Na2CO3 + 2H2 → 2NaOH + H2CO3
2NaOH + Cu(OH)2 → Na2SO4
Cu(OH)2 → CuO + H2O
D-glucose + 2CuO → D-gluconic acid + Cu2O (red ppt)
Requirements
Reagents
- Benedict’s reagent: Benedict’s reagent is prepared by adding 17.3 gm of sodium citrate, 10 gm of sodium carbonate and 17.3 gm of sodium pentahydrate to 100 ml of water in a beaker.
- Test samples
Materials required
- Test tube
- Test tube stand
- Pipettes
Equipment
- Water bath
Procedure
- About 1 ml of the test sample is added to a test tube along with 2 ml of benedict’s reagent.
- The test tubes are then placed in the test tube stand, which is kept in boiling water for 4-10 minutes.
- The color in the test tubes are observed and noted down.
Result and Interpretation
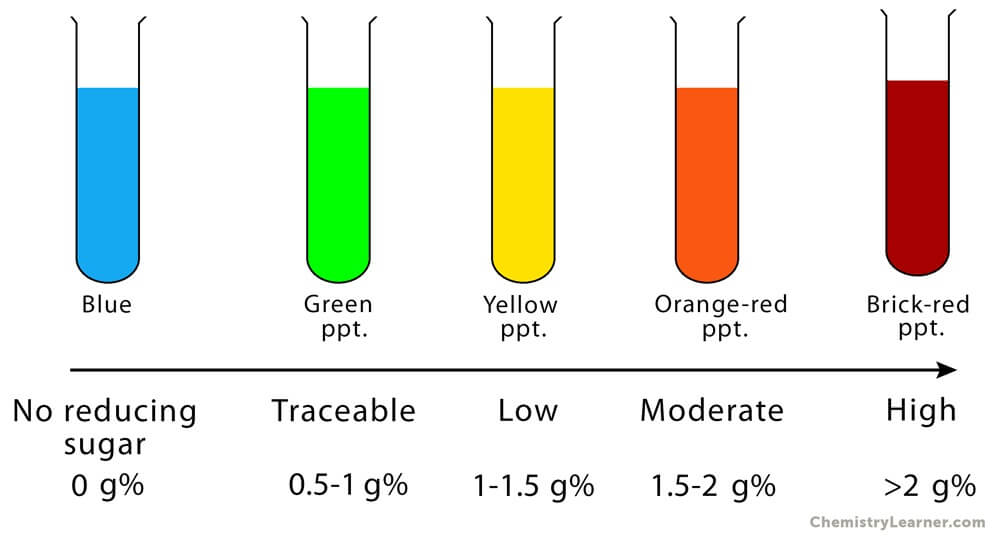
Figure: Observation (Results) of Benedict’s Test. Image Source: Chemistry Learner.
- The appearance of a greenish precipitate indicates about 0.5 g% concentration; yellow precipitate indicates 1 g% concentration; orange indicates 1.5 g% concentration and red indicates 2 g% or higher concentration of reducing sugars.
- The appearance of the blue color indicates the absence of reducing sugar and represents a negative result.
Notes
- Sucrose, starch, inositol gives a negative result, whereas lactose and maltose give a positive result with benedict’s test.
- Benedict modified the Fehling’s solution to make a single improved reagent, which is quite stable. It is very sensitive to even small quantities of reducing sugars (0.1%) and yields enough precipitate.
- A false-positive reaction for urine sample may be obtained due to the presence of reducing substances like uric acid, ascorbic acid or other drugs like levodopa.
Iodine test for starch
Definition
Iodine test, also known as a starch-iodine test, is a chemical test used to distinguish mono- or disaccharides from specific polysaccharides like amylase, dextrin, and glycogen.
Objectives
- To detect the presence of polysaccharide, primarily starch.
Principle
- Iodine test is based on the fact that polyiodide ions form colored adsorption complex with helical chains of glucose residue of amylase (blue-black), dextrin (black), or glycogen (reddish-brown).
- Monosaccharides, disaccharides, and branched polysaccharides like cellulose remain colorless. Amylopectin produces an orange-yellow hue.
- The reagent used in the iodine test is Lugol’s iodine, which is an aqueous solution of elemental iodine and potassium iodide.
- Iodine on its own is insoluble in water. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form triiodide ion, which further reacts with an iodine molecule to form pentaiodide ion.
- Bench iodine solution appears brown, whereas, the iodide, triiodide, and pentaiodide ion are colorless.
- It is observed that the helix (coil or spring) structure of the glucose chain is the key to this test.
- Further, the resulting color depends on the length of the glucose chains.
- The triiodide and pentaiodide ions formed are linear and slip inside the helix structure.
- It is believed that transfer of charge between the helix and the polyiodide ions results in changes in the spacing of the energy levels, which can absorb visible light, giving the complex its color.
- The intensity of the color decreases with the increase in temperature and the presence of water-miscible organic compounds like ethanol.
- On heating, the blue color amylase-iodine complex dissociates but is formed again on cooling because the helical structure is disrupted; thereby amylose loses its iodine binding capacity and the blue color.
- The blue color reappears on cooling due to the recovery of iodine binding capacity due to the regaining of the helical structure.
Requirements
Reagent
- Lugol’s iodine: 5% elemental iodine is mixed with 10% potassium iodide to form the Lugol’s iodine.
- Test sample
Materials Required
- Test tubes
- Test tube stand
Equipment
- Water bath
Procedure
- 1 ml of the test sample is taken in a test tube, to which 2-3 drops of Lugol’s reagent is added.
- The solution is then mixed in a vortex.
- The color of the solution is observed. The test tubes are then placed in the boiling water bath until the color disappears.
- The tubes are then cooled, and the color of the solution is observed and noted down.
Result and Interpretation

Figure: Observation (Results) of Iodine test for starch. Source: Comprehensive Natural Science (CNS).
- The appearance of blue-black or purple color represents a positive test, indicating the presence of starch.
- If there is no change in color, the result is negative and indicates the absence of starch.
Notes
- This test cannot be performed under acidic conditions as the starch hydrolyses under such circumstances.
- This test is a qualitative test and doesn’t signify the concentration of starch.
Emulsion test for Lipids
Definition
Emulsion test, also known as the Ethanol Emulsion test, is a general group test for the detection of lipids.
Objectives
- To determine the presence of lipids in a sample.
Principle
- The presence of lipids is observed by the appearance of a cloudy white layer on top of the reaction mixture.
- This test is based on the fact that lipids dissolve in ethanol (due to hydrophobic interaction), but on the addition of water, lipids spontaneously disperse to form micelles (small droplets).
- These droplets form the top layer as these are less dense than water and ethanol, and also appear cloudy white as they diffract light.
- The lipids come out of the solution because the overall strength of hydrogen bonding interaction between ethanol and water is much higher than hydrophobic interactions between lipids and ethanol.
Requirements
Reagents
- Ethanol
- Water
Materials required
- Test tubes
- Test tube stand
- Pipettes
Procedure
- Few drops of fat or lipid sample are added to a test tube. 2 ml of ethanol is added to the same test tube.
- To this solution, 2 ml of water is added, and the test tube is shaken well.
- The appearance of cloudy suspension is observed.
Result and Interpretation

Figure: Positive Results of Emulsion test for Lipids. Source: Tinycards.
- The appearance of cloudy suspension at the top layer of the solution indicates a positive result. This represents the presence of lipids in the sample. Samples with high lipid content will form a thicker cloudy suspension.
- The absence of cloudy emulsion indicates a negative result and the absence of lipid.
Biuret test for proteins
Definition
Biuret test, also known as Piotrowski’s test, is a chemical test for the detection of peptide bonds in a sample and can also be used for the quantification of proteins already in solution or easily soluble in dilute alkali.
Objectives
- To detect the presence of proteins or peptide bonds in a sample.
- To determine the concentration of proteins present in a sample.
Principle
- The biuret reagent contains sodium hydroxide, copper (II) sulfate, and potassium sodium tartrate.
- Under alkaline conditions of the biuret reaction (pH 14), deprotonation of the amide nitrogen occurs which leads to high electron density at the nitrogen atom,
- Further, copper (II) ion complexes with four peptide nitrogens to yield a tetradentate violet colored coordination complex.
- At high pH, Cu2+ bonding with OH– ion leads to an insoluble precipitate, which is minimized by the addition of potassium sodium tartrate, which stabilizes the cupric ions.
- Since peptide bonds occur with the same frequency per amino acid in proteins, the biuret test can be used to assess the concentration of proteins.
Requirements
Reagent
- Biuret reagent: 0.3 g of CuSO4 and 0.9 g of sodium-potassium tartrate are added to 50 ml of 0.2N NaOH. To this, 0.5 g of KI added and the volume is made up to 100 ml by adding 0.2N NaOH.
- Sample
Materials required
- Test tubes
- Test tube stand
- Pipettes
Equipment
- UV spectrophotometer
- Vortex
Procedure
- 1 ml of the sample is taken in a test tube to which few drops of Biuret reagent is added.
- The test tube is then mixed well by shaking the test tube well.
- The change in color of the solution is then observed and noted down.
Result and Interpretation

Figure: Results of the Biuret test for proteins.
- The appearance of the purple color of the solution indicates a positive result. The purple color present about 5-160 mg/ml concentration of proteins.
- The absence of the purple color indicates a negative result and the absence of proteins in the sample.
Notes
- The color developed in this test is stable, but it is recommended to take the readings of the sample within 10 minutes.
- For significant measurable color, peptides with at least three amino acids are necessary.
- Biuret is not a reagent in this test, but the test is so named because the reaction was first discovered with peptide-like bonds in the biuret molecule. A more accurate name for the reagent is the alkaline copper reagent (ACR) test.
References and Sources
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- 10% – https://www.researchgate.net/profile/Anand_Tiwari_Phd/publication/313745155_Practical_Biochemistry_A_Student_Companion/links/58ab15f04585150402034e42/Practical-Biochemistry-A-Student-Companion.pdf
- 2% – https://www.slideshare.net/soniherat/chemistry-of-carbohydrate-for-mbbs-students
- 1% – https://fac.ksu.edu.sa/sites/default/files/4_carbohydrate-i_.pdf
- 1% – https://en.wikipedia.org/wiki/Starch_indicator
- 1% – https://en.wikipedia.org/wiki/Emulsion_test
- 1% – https://en.wikipedia.org/wiki/Biuret_test
- 1% – https://en.wikipedia.org/wiki/Benedict%27s_reagent
- 1% – http://www.gmcsurat.edu.in/lib/exe/fetch.php?media=biochemistry:2017_august_ug_journal.pdf
- <1% – https://www.slideshare.net/shazi5250/identification-of-sugars-by-molisch-1
- <1% – https://www.scribd.com/doc/271555590/205177386-Comprehensive-Pharmacy-Notes
- <1% – https://www.scientificamerican.com/article/mix-it-up-with-oil-and-water/
- <1% – https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/potassium-sodium-tartrate
- <1% – https://www.researchgate.net/post/How_to_measure_the_concentration_of_starch
- <1% – https://www.quora.com/How-is-the-biuret-test-for-proteins-explained
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3529773/
- <1% – https://www.biochemden.com/color-reactions-of-lipids/
- <1% – https://www.answers.com/Q/Can_an_iodine_test_distinguish_between_amylose_and_amylopectin
- <1% – https://quizlet.com/35790284/microbiology-flash-cards/
- <1% – https://quizlet.com/10484544/urinalysis-quiz-2-flash-cards/
- <1% – https://en.wikipedia.org/wiki/Iodine_test
- <1% – https://diabetestalk.net/blood-sugar/what-colour-does-benedicts-solution-change-to-when-glucose-is-present
- <1% – https://biology-igcse.weebly.com/food-test-2—benedicts-test-for-reducing-sugars.html
- <1% – https://answers.yahoo.com/question/index?qid=20100109140646AA5zkua
- <1% – https://allmedtests.com/iodine-test-starch/
- <1% – http://www.jbc.org/content/5/5/485.full.pdf
- <1% – http://samson.kean.edu/~breid/enzyme/enzyme.html

WELL DONE. THANK YOU