Interesting Science Videos
What is Streptococcus mitis?
Streptococcus mitis is a Gram-positive coccus belonging to the viridians group of Streptococci as well as the mitis group.
- It is a commensal organism that colonizes different areas of the human body like the oropharynx, skin, and gastrointestinal and genital tract as a part of the normal flora.
- It is an important species of the genus Streptococcus consisting of are spherical or ovoid cells, arranged in chains or pairs.
- However, it has been associated with different diseases and infections of the human body as the organism acts as an opportunistic pathogen in the bodies of immunocompromised individuals.
- Besides, it was also found to be one of the major causative agents of viridans streptococci-related bacteremia and infective endocarditis.
- The genus name ‘mitis’ has been derived from the Latin term ‘mitis’ meaning mild, indicating that it is an organism with low pathogenicity and virulence that is involved in different types of mild infections.
- It was first isolated and discovered by Andrewes and Horder in 1906 from the human oropharynx region.
- S. mitis is the primary species of the mitis group consisting of twelve other species including the highly pathogenic S. pneumoniae.
- The species are put under the mitis group of Streptococci on the basis of their 16S rRNA gene sequences and nucleic acid hybridization data.
Classification of Streptococcus mitis
- The members of the genera Streptococcus belong to the group of lactic acid bacteria, which is a taxonomically diverse group of gram-positive, non-spore-forming cocci and rods defined by the formation of lactic acid as a sole or major endproduct of carbohydrate metabolism.
- The family Streptococcaceae is classifies based on 16S rRNA gene sequence analysis within the low (< 50 mol%) G+C branch of the Gram-positive eubacteria.
- The genus currently consists of over 50 recognized species, most of which fall within “species groups” which are identified on the basis of their 16S rRNA gene sequences.
- S. mitis is one of the pioneer species of the mitis group which has been poorly classified with several synonyms being applied to the same species.
- However, a new, more defined taxonomic description of the group has been made as a result of numerous phenotypic and genetic studies.
| Domain: | Bacteria |
| Phylum: | Firmicutes |
| Class: | Bacilli |
| Order: | Bacillales |
| Family: | Streptoococcaceae |
| Genus: | Streptococcus |
| Species: | S. mitis |
Habitat of Streptococcus mitis
- Streptococcus mitis is a commensal bacteria that mostly colonize the oral cavity along with the hard surfaces like the teeth and the mucosal membrane as a part of the oral flora.
- Besides, instances of S. mitis colonizing the gastrointestinal and the genital tract have also been studied.
- Streptococcus mitis is considered a typical representative organism of the commensal microbiota of the respiratory tract.
- The bacteria colonize several surfaces in the oral cavity and pharynx after birth and remains a numerically significant colonizer of those ecosystems throughout life.
- S. mitis comprise the majority of the earliest, “pioneer” species to colonize the mouths of healthy human neonates, along with S. oralis.
- Along with a few other Streptococcus species, it participates in the initial colonization of tooth enamel and may be present on nursing bottles and root surface caries.
- The oral cavity is a dynamic environment that undergoes large and rapid fluctuations in pH, nutrient availability and source, oxygen tension, temperature, and osmolality and the organism residing in such areas tend to have mechanisms to deal with such fluctuations.
- Members of S. mitis may cause infection when introduced into normally sterile compartments of the body or in immunocompromised patients.
- The optimum temperature for the organism is the average body temperature of the host, but it is known to survive in a temperature range of 18-40°C.
Morphology of Streptococcus mitis
- The cells of S. mitis are Gram-positive, oval, or elliptical in shape with an average diameter of 0.6 to 0.8 µm.
- The cells are arranged in chains, like all other Streptococci, but the cells are frequently observed in pairs and short chains. Longer chains are observed when grown in agar media.
- The arrangement of Streptococci is the result of successive division planes that are parallel to one another as in rod-shaped bacteria.
- The organism is catalase-negative, facultative anaerobe, not capsulated, and usually carries sparsely distributed, long fibrils on the surface.
- Extracellular surface structures and a variety of ell appendages of different lengths frequently occur in most strains. The density of the structures and the appendages differs with strains.
- The cell wall is composed of peptidoglycan, C-polysaccharide, and teichoic acid. The peptidoglycan type is Lys-direct.
- As in other gram-positive cell walls, the peptidoglycan consists of multiple glycan chains that are cross-linked through short peptides, and the glycan moiety is composed of alternating β-1,4-linked units of N-acetylglucosamine and N-acetylmuramic acid.
- The cell wall contains ribitol teichoic acid and lacks significant amounts of rhamnose. It contains phosphorylcholine residues in the teichoic acids of its cellular envelope.
- There are various cell-wall-associated surface proteins present on the cell wall that aid in the binding of the organism to different host surfaces.
- Underneath the cell wall is a cell membrane composed of the lipid-protein bilayer, along with different transport mechanism for the movement of molecules in and out of the cell.
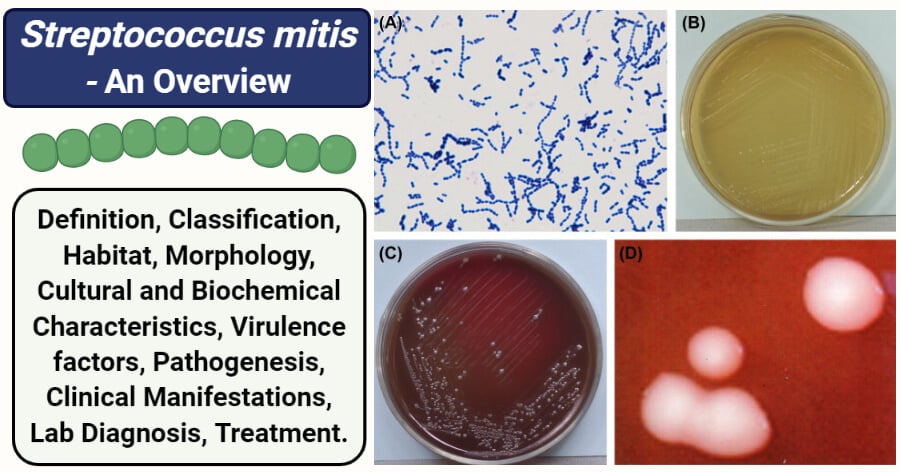
Figure: (A) Cells of S. mitis (Gram stain). (B) Colonies of S. mitis (TPY agar plate). (C) Colonies of S. mitis (BHI agar plate). (D) Colonies of S. mitis (stereomicroscope). Image Source: Atlas of Oral Microbiology (ScienceDirect).
Cultural Characteristics of Streptococcus mitis
- The growth of S. mitis is comparatively poor in general media like nutrient agar and thus require the addition of some antimicrobials and carbohydrate to make the media more selective for the organism.
- Media supplemented with blood, sucrose, and serums show more profuse growth.
- Blood agar and Chocolate agar are commonly used in the identification of S. mitis to observe the hemolysis.
- For more selective isolation, media like Brain Heart Infusion Agar and Trypticase soy agar/broth with defibrinated sheep blood can be used.
- It is a facultative anaerobe, so abundant growth is observed in the air with 5% carbon dioxide at 37°C.
- Most strains are unable to grow in the presence of 6.5% NaCl, whereas a few strains may grow in 4% NaCl.
1. Nutrient Agar
- White to grey colored colonies of an average size of 1 mm in diameter. The colonies were round with raised elevation and an entire margin.
- Growth is mostly poor and requires air with supplied carbon dioxide.
2. Blood agar
- Typical smooth, non-pigmented, convex colonies with entire margin are observed on blood agar.
- Growth occurs readily on blood agar and exhibits various types of hemolysis, but mostly α-hemolysis. About 1-2 mm of a green-colored zone of hemolysis is observed on blood agar.
- Pronounced greening is observed on chocolate agar.
Biochemical characteristics of Streptococcus mitis
The biochemical characteristics of S. mitis can be tabulated as follows:
| S.N | Biochemical Characteristics | Streptococcus mitis |
| 1. | Capsule | No capsule |
| 2. | Shape | Cocci |
| 3. | Catalase | Negative (-) |
| 4. | Oxidase | Positive (+) |
| 5. | Citrate | Negative (-) |
| 6. | Methyl Red (MR) | Positive (+) |
| 7. | Voges Proskauer (VR) | Negative (-) |
| 8. | OF (Oxidative-Fermentative) | Facultative anaerobes |
| 9. | Coagulase | Negative (-) |
| 10. | DNase | Negative (-) |
| 11. | Clumping factor | Negative (-) |
| 12. | Gas | Negative (-) |
| 11. | H2O2 | Positive (+) |
| 12. | Hemolysis | α-hemolytic |
| 13. | Motility | Non-motile |
| 14. | Nitrate Reduction | Negative (-) |
| 15. | Gelatin Hydrolysis | Negative (-) |
| 16. | Pigment Production | Variable |
| 17. | Bile esculin test | Negative (-) |
| 18. | Ig A1 protease | Positive (+) |
| 19. | Urease | Negative (-) |
| 19. | Lancefield group | Some strains react with Group K and O antisera while others are non-groupable. |
Fermentation
| S.N | Substrate | Streptococcus mitis |
| 1. | Glucose | Positive (+) |
| 2. | Fructose | Positive (+) |
| 3. | Galactose | Positive (+) |
| 4. | Lactose | Positive (+) |
| 5. | Maltose | Variable |
| 6. | Mannitol | Negative (-) |
| 7. | Mannose | Positive (+) |
| 8. | Raffinose | Variable |
| 9. | Ribose | Variable |
| 10. | Sucrose | Positive (+) |
| 11. | Starch | Negative (-) |
| 12. | Trehalose | Positive (+) |
| 13. | Xylose | Negative (-) |
| 14. | Salicin | Variable |
| 15. | Glycerol | Negative (-) |
| 16. | Dulcitol | Negative (-) |
| 17. | Cellobiose | Positive (+) |
| 18. | Rhamnose | Negative (-) |
| 19. | Arabinose | Negative (-) |
| 20. | Inulin | Negative (-) |
| 21. | Sorbitol | Variable |
| 22. | Pyruvate | Negative (-) |
| 23. | Glycogen | Negative (-) |
Enzymatic Reactions
| S.N | Enzymes | Streptococcus mitis |
| 1. | Acetoin | Negative (-) |
| 2. | Acid Phosphatase | Variable |
| 3. | Alkaline Phosphatase | Positive (+) |
| 4. | Ornithine Decarboxylase | Not determined |
| 5. | Hyaluronidase | Negative (-) |
| 6. | β-D-glucosidase | Positive (+) |
| 7. | Leucine aminopeptidase | Positive (+) |
| 8. | Neuraminidase | Positive (+) |
Virulence factors of Streptococcus mitis
- S. mitis has generally been considered a relatively benign oral streptococcus and member of the oral commensal flora.
- Nevertheless, S. mitis is involved in a range of invasive disease in humans and lately, has emerged as a cause of bloodstream infections in immune-compromised patients, and in patients undergoing cytotoxic anti-cancer chemotherapy.
- S. mitis is considered a leading cause of infective endocarditis and bacteremia, among the oral streptococci
- Very few studies have been done regarding the role of S. mitis virulence factors, which is why there is minimal information about the streptococcal virulence factors and their roles in disease pathogenesis.
- Some of the known and studied virulence factors of S. mitis that is involved in the pathogenesis of diseases are:
1. Phage proteins
- The binding of S. mitis to human platelets contributes to the pathogenesis of S. mitis infective endocarditis.
- Platelet binding by S. mitis is mediated in part by two bacteriophages encoded proteins, PblA and PblB.
- Both PblA and PblB produced by the bacteriophage mediate the attachment of the bacteriophage to the choline residues present on the cell walls of viable bacteria where they then enable the binding of viable bacteria to platelets on the host.
- The sialic acid of the platelet membrane ganglioside is the target receptor for the phage-encoded proteins PblA and PblB.
- Other virulence factors, in addition to PblA and PblA that mediate the binding of the bacteria to platelets, are yet not wholly understood.
2. Immunoglobulin A1 protease
- Streptococcus mitis can produce an IgA1 protease, which is homologous to the IgA1 proteases of S. oralis.
- These proteases are cell-wall-anchored zinc metalloproteases that cleave peptide bonds in IgA1.
- This proteolytic cleavage of IgA1 by the protease generates Fab and Fc fragments which can dissociate the recognition of S. mitis antigens from mechanisms for their elimination.
- In addition to this, the remaining bacterial bound Fab fragments could mask the epitopes from the immune system and prevent the binding of other antibody isotypes, activation of complement, and complement-mediated lysis.
3. Wall-associated surface proteins
- There are about 18 predicted cell-wall-associated surface proteins bearing the cell wall attachment motif LPXTG encoded within the genome of S. mitis.
- Some of these proteins include the NanA2 protein that binds to sialic acid of the platelets and MonX, which is a platelet-binding adhesion.
- These proteins are involved in the colonization of the host surfaces and pathogenesis of the disease.
4. Cytolysin
- Streptococcus mitis does not produce a wide range of toxins, but it has been shown to encode and produce a toxin, which is structurally and functionally similar to the S. pneumoniae pneumolysin and the S. intermedius intermedilysin.
- The S. mitis -specific toxin, named mitilysin, is functionally similar to pneumolysin in hemolytic assays and cross-reacts with pneumolysin antibodies.
- However, this cytotoxin has only been identified in a few strains of S. mitis, and studies related to its role in the pathogenesis of the infections have yet not been done.
Pathogenesis of Streptococcus mitis
Streptococcus mitis is one of the pioneer colonizers of the neonatal human oropharynx and is a numerically significant commensal throughout life. However, it is also considered one of the important pathogenic agents among the viridians streptococci causing infections like meningitis and infective endocarditis. Different virulence factors expressed by the organism aid in the process of pathogenesis.
1. Transmission
- S. mitis is a part of the human normal flora, but its origin remains yet to be determined.
- However, it has been known that commensal bacteria transfer from the external environment, the primary caregiver, and from other areas of the respiratory tract after birth.
- The bacteria, however, might reach sterile parts of the body like the brain and the heart via blood in the case of infection.
2. Adhesion/ Attachment/ Colonization
- Successful colonization of the oropharynx is dependent on a number of different factors.
- Initially, S. mitis expresses several adhesins that promote primary attachment to host tissues.
- The most important proteins involved in the binding of the organism to the cell surface are the wall-associated adhesins or proteins that bind to different types of cells.
- Proteins like NanA2 are involved in the binding of the bacterium to the sialic acid residues of the epithelial cell.
- Analysis of its genome sequence confirmed that S. mitis encodes numerous cell wall-anchored and choline-binding proteins that are effective adhesins that help in attachment and colonization.
3. Invasion
- Some virulent strains of S. mitis produce a cytotoxin, mitilysin that lyses the epithelial cells and makes its way into the host circulatory system.
- In the blood, the phage proteins PblAand PblB substantially contribute to the adherence of S. mitis to platelets.
- Via platelets, S. mitis reaches different parts of the body where it causes different diseases.
4. Interaction with the immune system
- Following primary attachment, S. mitis employs several strategies to escape the innate and acquired immune systems of the host.
- The saliva in the oral cavity contains secretory immunoglobulin A (IgA) antibodies that react with S. mitis. However, these antibodies do not entirely block adherence and subsequent colonization.
- S. mitis can produce an IgA1 protease that cleaves peptide bonds in IgA1, separating the Fab and Fc regions.
- This prevents the binding of the antibody and other immune system mechanisms.
- Besides, the tissue destruction associated with disease caused by S. mitis is mostly mediated by the host inflammatory response to infection by the bacteria.
- S. mitis can also modify the expression of the proinflammatory chemokine interleukin-8, which results in the destruction of the tissues and disease occurrence.
Clinical manifestations of Streptococcus mitis
- In the majority of people, S. mitis is a commensal bacteria of the oral cavity that presents no significant immunological threat.
- However, S. mitis has emerged as a significant pathogen in elderly patients, immunocompromised patients, and in patients undergoing cytotoxic chemotherapy treatment for cancer.
- Streptococcus mitis is also an infrequent opportunistic pathogen in normal healthy infants and adults known to cause a wide range of diseases from dental caries, to bacterial infective endocarditis, bacteremia, meningitis, eye infections, and pneumonia.
- Besides, S. mitis has also been implicated as an etiologic agent in different urinary tract infections.
- S. mitis is also rarely associated with septic arthritis, which if left undiagnosed, might result in an increased risk for permanent bone damage or septicemia.
Lab Diagnosis of Streptococcus mitis
The diagnosis of S. mitis from clinical specimens is primarily involved in the identification of the organism from these specimens. Depending on the site of infection, different samples are taken for diagnosis. For oral infection, swabs and dental plaques are collected, whereas for urinary tract infections, urine is collected.
The following are different types of diagnostic methods that can be employed for the correct isolation and identification of S. mitis:
1. Morphological, cultural, and biochemical characteristics
- Oral streptococci can often be isolated on selective media where colony morphology provides the first basis for the identification of the organism.
- The appearance of typical smooth, non-pigmented, convex colonies with an entire margin on blood agar with α-hemolysis indicates the presence of S. mitis.
- Isolation is then followed by the microscopic observation of the organism for the cell morphology and arrangement.
- The appearance of Gram-positive, non-motile, non-spore-forming cocci in pairs or short chains provide further basis for the presence of S. mitis.
- Biochemical tests are then performed for the species determination and confirmation of the organism.
- Lancefield antigen grouping can also be performed as some strains of S. mitis react to K and O antisera while others are non-groupable.
2. Rapid diagnosis
- Besides the traditional methods of species identification, commercial rapid identification kits for species identification of Streptococcus is also available now.
- Commercial kits such as Rapid Strep 32 can be used for the identification of Streptococcus species.
- In the case of S. mitis, the identification is based on the analysis of their microbial cellular fatty acid compositions.
3. Molecular diagnosis
- Determination of 16S rRNA sequence is the most critical molecular method for the confirmation of S. mitis.
- Besides, tests like PCR and DNA sequencing can be performed for a more accurate confirmation and diagnosis.
- To some extent, identification may also be achieved with DNA probes that hybridize exclusively with the individual species.
Treatment of Streptococcus mitis infections
- Because infections caused by S. mitis are mostly mild (except infective endocarditis), treatment of the disease is mostly easy.
- Most strains of S. mitis are susceptible to different group of antibiotics, however recent cases of resistance is observed against penicillin, erythromycin, and, rarely, clindamycin.
- The primary antibiotic treatment for older patients (>50 years) against meningitis caused by S. mitis is ceftriaxone 4 g/day and ampicillin 12 g/day.
- In the case of medical-device related infections like endocarditis, removal, or replacement of the device might be required.
References
- Topley WWC (2007). Topley and Wison’s Microbiology and Microbial Interactions; Bacteriology, 2 Vol. Tenth Edition. John Wiley and Sons Ltd.
- Bergey, D. H., Whitman, W. B., De, V. P., Garrity, G. M., & Jones, D. (2009). Bergey’s manual of systematic bacteriology: Vol. 3. New York: Springer.
- Streptococcus mitis (ATCC® 49456™).
- Mitchell J. Streptococcus mitis: walking the line between commensalism and pathogenesis. Mol Oral Microbiol. 2011 Apr;26(2):89-98. doi: 10.1111/j.2041-1014.2010.00601.x. Epub 2011 Jan 18. PMID: 21375700.
- Andrew Smith, Margaret S. Jackson & Helen Kennedy (2004) Antimicrobial susceptibility of viridans group streptococcal blood isolates to eight antimicrobial agents, Scandinavian Journal of Infectious Diseases, 36:4, 259-263, DOI: 10.1080/00365540410019435
- Rasmussen, Louise & Højholt, Katrine & Dargis, Rimtas & Christensen, Jens Jørgen & Skovgaard, Ole & Justesen, Ulrik & Rosenvinge, Flemming & Moser, Claus & Lukjancenko, Oksana & Rasmussen, Simon & Nielsen, Xiaohui. (2017). In silico assessment of virulence factors in strains of Streptococcus oralis and Streptococcus mitis isolated from patients with Infective Endocarditis. Journal of medical microbiology. 66. 10.1099/jmm.0.000573.
- Hohwy, J., Reinholdt, J., & Kilian, M. (2001). Population dynamics of Streptococcus mitis in its natural habitat. Infection and immunity, 69(10), 6055–6063. https://doi.org/10.1128/IAI.69.10.6055-6063.2001
- Selda Sayin Kutlu, Suzan Sacar, Nural Cevahir, Huseyin Turgut, Community-acquired Streptococcus mitis meningitis: a case report, International Journal of Infectious Diseases, Volume 12, Issue 6, 2008, Pages e107-e109, ISSN 1201-9712, https://doi.org/10.1016/j.ijid.2008.01.003.
- Fatma Al-Farsi, Ibrahim Al-Busaidi, Khalfan Al-Zeedi, “Acute Streptococcus mitis Sacroiliitis in a Teenager with Unclear Source of Bacteremia: A Case Report and Literature Review”, Case Reports in Infectious Diseases, vol. 2018, Article ID 2616787, 3 pages, 2018. https://doi.org/10.1155/2018/2616787
- Oteo, Jesús M.D.; Avilla, José M.D.; Alós, Juan-Ignacio Ph.D.; Gómez-Garcés, José-Luis M.D., Ph.D. URINARY TRACT INFECTION CAUSED BY STREPTOCOCCUS MITIS HIGHLY RESISTANT TO PENICILLIN, The Pediatric Infectious Disease Journal: July 1997 – Volume 16 – Issue 7 – p 724-725.
- Kutlu, S. S., Sacar, S., Cevahir, N., & Turgut, H. (2008). Community-acquired Streptococcus mitis meningitis: a case report. International Journal of Infectious Diseases, 12(6), e107–e109. doi:10.1016/j.ijid.2008.01.003.

My son is diagnosed recente with endocarditis caused by streptococcus mitis oralis. Hé is Marfan patiënt and has an artificial heartprothesis.
Is there new information available of how to treat with which antibioticum?