Interesting Science Videos
Salt Saturation Test Definition
Salt Saturation Test is a test for the precipitation of proteins and their differentiation from other proteins by using the salting-out technique. Salting out is the process of precipitation of proteins by increasing the concentration of salt in the solution. The salts used in this technique are mostly neutral mineral salts like MgSO4, Na2SO4, and (NH)2SO4. The salt solubilization test also helps in the differentiation of proteins as some proteins precipitate under lower salt concentrations while others require higher concentrations. The reagent used for the test might differ depending on the preference with salts like ammonium sulfate being used due to their increased solubility. The salt saturation test can be performed either as half saturation or full saturation depending on the type of proteins being detected. In the half-saturation, the solution is just half saturated with the salt, but in full saturation, the solution is completely saturated with the salt.
Objectives of Salt Saturation Test
- To detect the presence of proteins in a given sample.
- To differentiate between proteins like albumin and globulin.
- To purify and fractionate large batches of proteins based on their solubility.
Principle of Salt Saturation Test
Proteins are colloidal in nature due to the presence of a large number of electric charges which creates repulsion, preventing the coalescence of the particles. Similarly, a shell of hydration is also formed around each protein molecule where the proteins are surrounded by films of water molecules. The mechanism of the salting-out technique is the preferential solvation of salt molecules by the exclusion of the hydration layer around the protein molecules. When organic salts are added to the solution, the effective water concentration available for the proteins decreases which eventually leads to the precipitation of the proteins. There are two different mechanisms involved in the process.
At first, the higher concentration of mineral salts osmotically removes water from the hydration layer around proteins, which then depletes the immobilized layer of water surrounding each of the protein particles. Secondly, the cations and anions of the mineral salts bind with the respective counterionic groups on the protein particles to reduce the surface charges of the proteins. Both of these mechanisms aid in the aggregation and precipitation of protein particles. The concentration of the salt required for the precipitation of proteins depends on the surface area of the proteins. Smaller molecules like albumin have a larger surface area which requires a higher concentration of salt, whereas larger molecules like casein, gelatin, and globulin have a smaller surface area and require smaller concentrations.
Requirements
Reagent
- Solid Ammonium sulfate
- Saturation solution of Ammonium sulfate
- 40% sodium hydroxide
- 1% copper sulfate solution
- Sample solution
Materials required
- Test tube
- Test tube stand
- Dropper
- Pipettes
- Filter paper
Procedure of Salt Saturation Test
For Half Saturation
- In a test tube, 3 ml of the test solution is taken. To this, 3 ml of the saturated solution of ammonium sulfate is added.
- The solution is mixed correctly and allowed to stand for about 5 minutes.
- The solution is then filtered to remove the residue formed from the rest of the filtrate.
- The Biuret test is performed on the rest of the filtrate.
Full Saturation
- In a test tube, 3 ml of the test solution is taken. To this, some amount of solid ammonium sulfate is added.
- The solution is then mixed by shaking until the solid salt dissolves. The solid ammonium sulfate is added continuously until no more of the salt can be dissolved.
- The solution is then allowed to stand for five minutes, followed by the filtration of the residue.
- The Biuret test is performed on the filtrate.
Result and Interpretation of Salt Saturation Test
Half Saturation
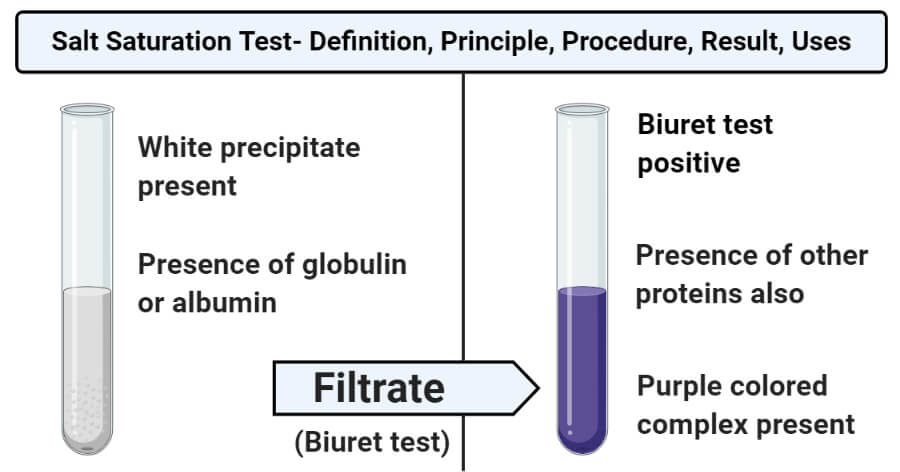
- The formation of a white precipitate in the solution under half-saturation indicates a positive result. This result confirms the presence of globulin in the solution.
- The absence of the white precipitate represents a negative result, indicating the absence of globulin in the sample.
- If a purple color is formed during the Biuret test, it shows that other proteins are present in the solution. A negative Biuret test indicates the absence of other proteins.
Full Saturation
- The formation of a white precipitate in the solution under full saturation indicates a positive result. This result confirms the presence of albumin in the solution.
- The absence of the white precipitate represents a negative result, indicating the absence of albumin in the sample.
- If a purple color is formed during the Biuret test, it shows that other proteins are present in the solution. A negative Biuret test indicates the absence of other proteins.
Uses of Salt Saturation Test
- Salt saturation test can be used for the detection of proteins like albumin and globulin.
- As the precipitation of proteins in the test is due to reduced solubility and not denaturation, precipitated proteins can be used to detect the concentration of the proteins.
- The test can also be used as a method of protein purification for the removal of bound lipid or nucleic acids.
- The mineral salts used in the test help in the stabilization of proteins by preferential solvation. Salts like (NH)2SO4 inhibit bacterial growth and protease activity on the proteins as well.
Limitations of Salt Saturation Test
- While using solid ammonium sulfate, lumps might be formed in the solution. In order to avoid that, mortar and pestle should be used.
- The use of low-grade mineral salts might cause contamination with heavy metals, that inhibit the precipitation process.
- Online calculators can be used to determine the amounts of solid ammonium sulfate required for saturation.
- The use of ammonium sulfate might cause acidification of the solution, which slows down the process; thus, Tris buffer can be added.
References
- Wingfield, P. “Protein precipitation using ammonium sulfate.” Current protocols in protein science Appendix 3 (2001): Appendix 3F. doi:10.1002/0471140864.psa03fs13
- D (2012). Biochemistry. Fourteenth Edition. Academic Publishers. Kolkata.
- https://studylib.net/doc/5791302/19.-salt-saturation-test
- https://alevelbiology.co.uk/notes/test-for-proteins
