Interesting Science Videos
Sakaguchi Test Definition
Sakaguchi test is a biochemical test consisting of colorimetric reaction for the detection and quantification of guanidinium groups, used as a qualitative test for arginine that is either free or in protein. The test was discovered by and named after the Japanese Food Scientist Shoyo Sakaguchi in 1925. Sakaguchi test is an example of color reactions or test that is performed for the detection of amino acids or proteins. The test is a specific test for arginine where the guanidinium group of arginine reacts with 1-naphthol or α-naphthol to produce a colored product. It is a qualitative test, and the quantification of arginine is hindered in this test due to the slow rate of color development and destruction of some guanidinium groups by the reagent.
Objective of Sakaguchi Test
- To detect the presence of arginine in either free form or in proteins.
Principle of Sakaguchi Test
Sakaguchi test is based on the principle of reaction between 1-naphthol and the guanidinium groups in arginine, in the presence of an oxidizing agent. The exact mechanism of the reaction is not yet known; however, the reaction results in the formation of a red-colored complex due to the formation of an indole-like structure. The Sakaguchi reagent consists of sodium hypobromite and 1-naphthol. The sodium hypobromite acts as an oxidizing agent that facilitates the hydrogen bonding between two arginine molecules.
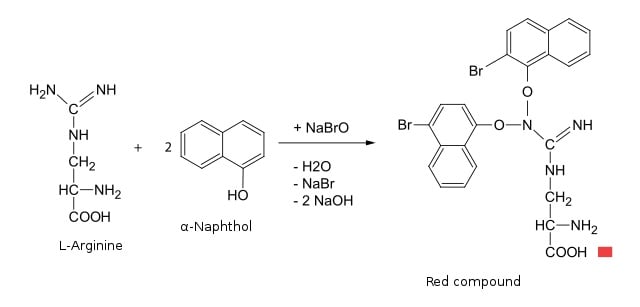
Image Source: Zlir’a.
Requirements
Reagent
- Sakaguchi reagent: 1% 1-naphthol in alcohol with a few drops of 10% sodium hypobromite solution of bromine water.
- 40% NaOH
- Sample (0.1% of arginine or 0.1% of creatine)
Material required
- Test tubes
- Test tube stand
- Pipettes
Procedure of Sakaguchi Test
- About 3 ml of the test solution is added in a test tube, to which 1 ml of 40% NaOH is added and mixed correctly.
- Then, two drops of 1-naphthol are added to the same test tube and mixed thoroughly.
- Now, 4-5 drops of the 10% sodium hypobromite or bromine water is added.
- The test tube is observed for the development of color.
Result and Interpretation of Sakaguchi Test
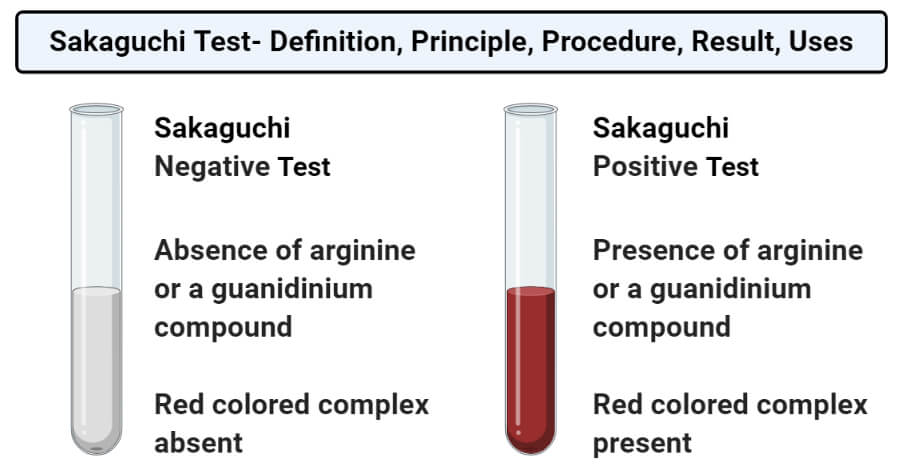
- Positive result: A positive result on the Sakaguchi’s test is demonstrated by the formation of red color. This indicates the presence of an arginine or guanidinium compound.
- Negative result: A negative result in the Sakaguchi’s test is demonstrated by the absence of red color. This indicates an absence of arginine or a guanidinium compound.
Uses of Sakaguchi Test
- Sakaguchi’s Test is a biochemical test for the detection of arginine in the free or combined form in proteins.
- The test is qualitative but can be made quantitative by the addition of urea that stabilizes the colored product.
Limitations
- Quantitative analysis of the colored product is not possible with this test as the rate of Sakaguchi’s reaction is slow.
- Similarly, some of the guanidinium groups in the solution might be destroyed by the hypochlorite, resulting in difficulties in testing results.
References
- Webner C.J (1929). A modification of Sakaguchi’s reaction to the quantitative determination of arginine. JBC. December 27.
- Ke, Shan, and Robert Haselkorn. “The Sakaguchi reaction product quenches phycobilisome fluorescence, allowing determination of the arginine concentration in cells of Anabaena strain PCC 7120.” Journal of Bacteriology 195,1 (2013): 25-8. doi:10.1128/JB.01512-12
- Tiwari, A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
