Respiratory Syncytial Virus (RSV) belonging to the Paramyxoviridae family is one of the most important causes of lower respiratory tract illness in infants less than 1 year of age.
Along with infants, individuals with compromised immune, pulmonary, or cardiac systems, and the elderly are also at high risk for RSV infection.
RSV infection can cause upper respiratory tract infection, and bronchiolitis, which can rarely lead to pneumonia, respiratory failure, apnea, and death.
The virus was first isolated in 1956 from nasal secretions of chimpanzees with rhinorrhea and coryza and was initially named “Chimpanzee coryza agent” (CCA).
Two major antigenic subgroups of RSV, A, and B, differ in the glycoproteins G and F and show epidemiological differences. Although the subtypes cocirculate, one subtype generally predominates the other depending on the region and climate.
As the clinical symptoms of the RSV infection overlap with other respiratory illnesses, laboratory tests are required for the diagnosis.
Interesting Science Videos
Structure of Respiratory Syncytial Virus (RSV)
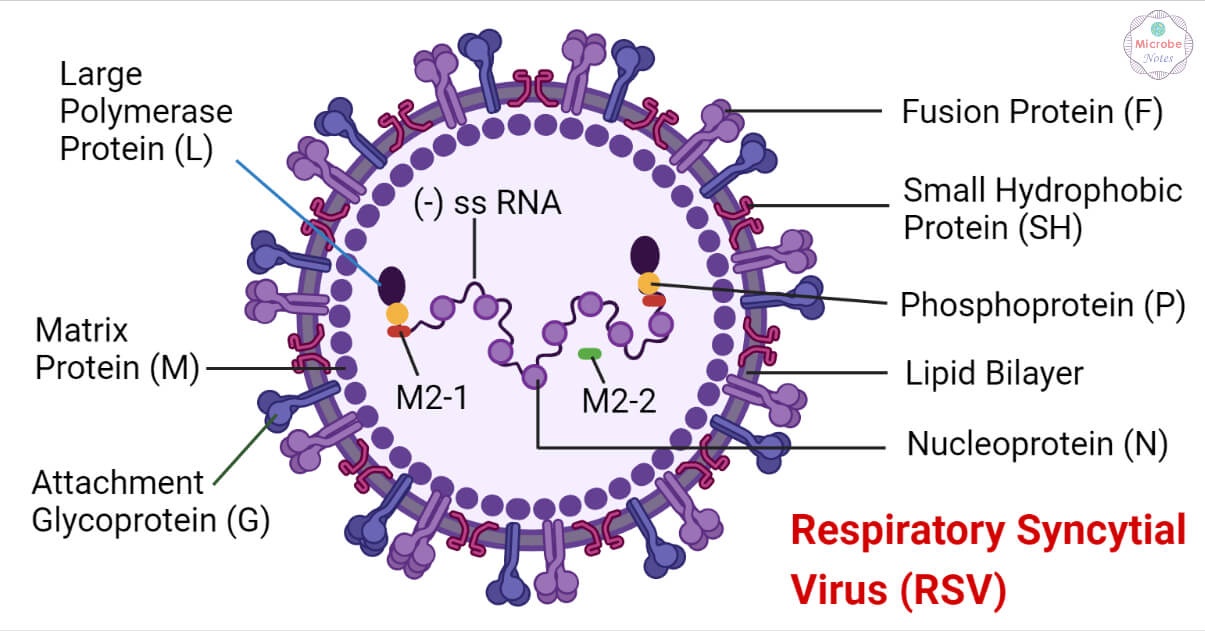
- The RSV virion consists of a nucleocapsid packaged in a bilipid-layer envelope derived from the host membrane.
- Virions cultured in cell lines consist of spherical particles of 100-350 nm in diameter and long filaments of 60-120 nm diameter and up to 10 μm in length.
- The virus envelope consists of a fusion protein (F), a small hydrophobic protein (SH), and the attachment protein (G).
- The viral glycoproteins form separate homo-oligomers that are presented as short surface spikes (11-16nm).
- The matrix protein (M) lies underneath the lipid envelope.
- The genome consists of a single-stranded, negative-sense RNA encapsulated by the nucleoprotein (N).
- The RNA polymerase protein (L), phosphoprotein (P), and the transcription processivity factor M2-1 also remain associated with the nucleocapsid.
Genome Structure of Respiratory Syncytial Virus (RSV)
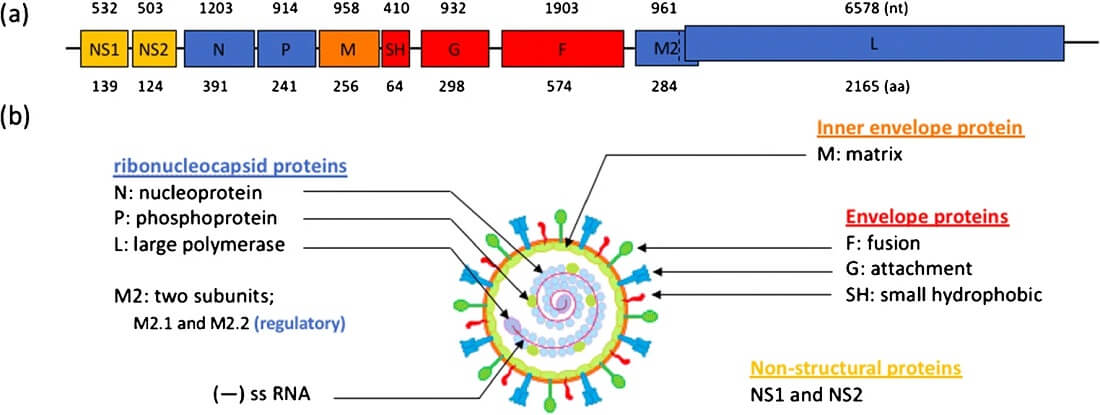
- The RSV genome consists of a single-stranded, negative-sense RNA having 10 open reading frames (ORFs).
- The RNA is approximately 15.2 kb in size.
- It encodes 11 structural and nonstructural proteins.
- A complimentary copy of the genome called the antigenome is involved in RNA replication.
- Both the genome and antigenome lack 5’ caps or 3’ polyA tails.
- It consists of a leader region and a trailer region in the 3’ and 5’ ends respectively.
- The RNA consists of the leader region, followed by NS1, NS2, N, P, M, SH, G, F, M2, L, and trailer region in order from 3’ to 5’ end.
- The RNA encapsidation protects it from degradation and shields it from recognition by host cell receptors that initiate immune responses.
Epidemiology of Respiratory Syncytial Virus (RSV)
- RSV is a widespread human pathogen that causes frequent reinfections.
- 90% of the children are infected with the virus within the first 2 years of age with frequent reinfections in elderly populations.
- Of all the children, 0.5% to 2.0% of them are hospitalized with lower respiratory tract infections, 50% to 90% develop bronchiolitis and 5% to 40% develop pneumonia.
- An estimated 33 million cases are reported annually in children with 3 million hospitalization and 120,000 deaths from complications associated with the infection.
- RSV infections show seasonal variations in different geographical areas of the world.
- Premature infants, patients with preexisting cardiac, pulmonary, neurologic, immunosuppressive disorders, and the elderly constitute the population at risk for morbidity and mortality.
- In Canada, RSV causes 5800 to 12,000 hospitalizations every year while 77,700 hospitalizations due to bronchiolitis were reported in the United States between 1997 to 2000.
- Underdeveloped countries show higher rates of mortality with an estimated 66,000 to 199,000 deaths occurring in children younger than 5 years of age in 2005.
Transmission of Respiratory Syncytial Virus (RSV)
RSV spreads from person to person via respiratory droplets through coughs or sneezes, virus droplets getting in the eyes, nose, or mouth, through fomites, and direct contact with the infected individual (e.g., kissing an infected person)
It has an incubation period of 2 to 8 days, generally, 4 to 6 days depending on various factors like the age of the patient and whether it is the primary infection of RSV of the patient.
Patients at high risk for developing severe diseases associated with RSV infection include:
- Infants younger than 6 months of age
- Infants and children with underlying lung diseases, such as bronchopulmonary dysplasia or congenital heart diseases
- Infants exposed to secondhand smoking
- Immunocompromised patients (e.g., patients with immune disorders, or who have recently undergone organ transplantation)
- Individuals with asthma
- Individuals with cardiopulmonary disease
- Elderly patients having a chronic obstructive pulmonary disease (COPD)
Replication of Respiratory Syncytial Virus (RSV)
- Attachment
The RSV attaches to its receptors like the chemokine receptor, CX3CR1, present on the apical surface of ciliated epithelial cells through the attachment glycoprotein (G protein).
- Fusion
The fusion protein (F) mediates the fusion of the viral membrane with the host membrane and releases the nucleocapsid into the host cell cytoplasm.
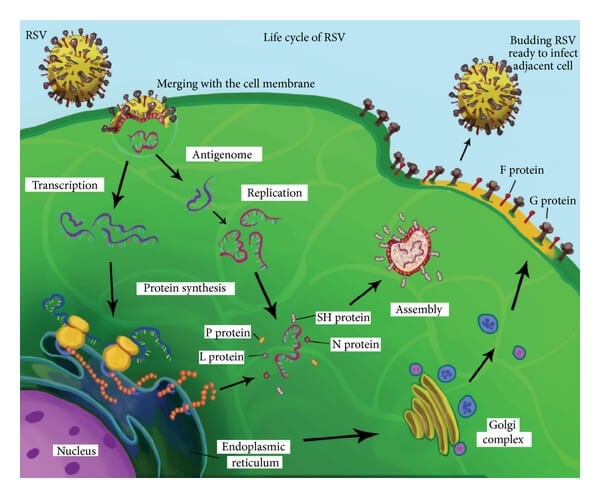
- Biosynthesis
The negative-sense RNA serves as a template for the RSV polymerase to synthesize mRNAs and genome progeny. Surface glycoproteins are produced, post-translationally modified, and transported through the endoplasmic reticulum to the host cell membrane. The structural and nonstructural proteins are synthesized through a translation of the mRNAs. Progeny of genome and antigenome is also synthesized.
- Assembly
The matrix protein (M) associates with the replication complex and interacts with the cytoplasmic tail of F protein to viral filaments allowing the viral nucleocapsid to be packaged into viral filament particles.
- Release
The RSV is released from the cells through the process of budding taking up the surface glycoproteins with the membrane that constitutes the viral envelope.
Pathogenesis of Respiratory Syncytial Virus (RSV)
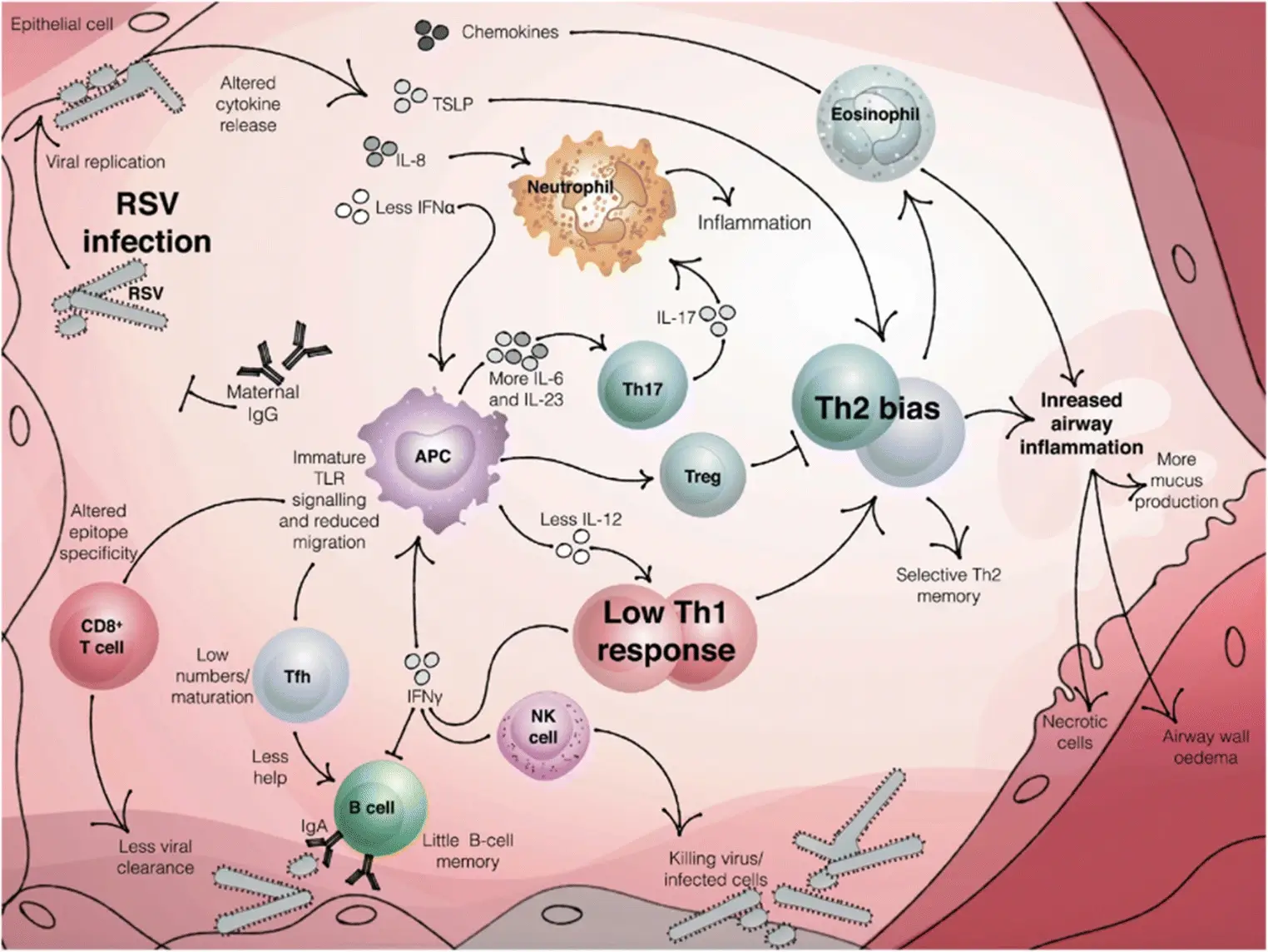
- RSV pathogenesis is a complex and variable process affected by many host and viral factors.
- The disease can range from mild rhinitis to severe diseases of the upper and lower respiratory tract like bronchiolitis and pneumonia.
- Although the virus can show direct cytopathology of respiratory epithelium, in the immunocompetent, the immune response plays a more important role in the disease pathogenicity.
- After the virus enters the host body through the transmission routes, the virus rapidly spread into the respiratory tract, where it multiplies in the apical ciliated epithelial cells.
- In typical RSV primary infection, host response is dominated by IFN-ϓ produced by NK, CD4+, and CD8+ T cells.
- RSV infection after immunization with FI-RSV or RSV G glycoprotein induces an immune response dominated by type 2 cytokines and is associated with lung eosinophilia and airway mucus production.
- RSV infection with allergic inflammation or absence of STAT1-mediated signaling induces airway epithelial mucus with the expression of IL-17 cytokine.
- Both the humoral and cytotoxic T-cell activation is triggered which results in viral cytotoxicity as well as cytotoxicity from the host’s immune response causing necrosis of respiratory epithelial cells.
- It can lead to small airway obstruction by mucus, cellular debris as well as alveolar obstruction.
- Other effects may include ciliary dysfunction with impaired mucus clearance, airway edema, and decreased lung compliance.
Clinical Manifestations of Respiratory Syncytial Virus (RSV)
The symptoms of RSV infection generally start about 4 to 6 days after infection. The usual symptoms of the infection include:
- Runny nose
- Decrease in appetite
- Cough
- Sneezing
- Fever
- Wheezing
These symptoms usually appear in stages and not all at once. In severe cases of infection that usually affects the lower respiratory tract, it can cause pneumonia or bronchiolitis. The signs and symptoms may include:
- Fever
- Severe cough
- Wheezing
- Rapid breathing or difficulty in breathing
- Cyanosis (bluish coloration of the skin due to lack of oxygen)
- Middle ear infection (otitis media)
Infants are more severely infected by RSV infection having symptoms such as:
- Short, shallow, and rapid breathing
- Struggling to breathe (chest muscles and skin pull inward with each breath)
- Cough
- Poor feeding
- Unusual tiredness
- Irritability
Most children recover within one to two weeks, although life-threatening infection may occur in infants having chronic heart or lung problems.
Diagnosis of Respiratory Syncytial Virus (RSV)
Rapid Antigen Detection Tests
Due to the ease of use, quick turnaround time, and acceptable sensitivity and specificity, the RADTs are popular for RSV testing. However, it can sometimes provide false-negative results and hence, a more sensitive method (e.g., PCR) must be considered in those cases.
Direct Fluorescent Antibody
Direct fluorescent antibody (DFA) is a fairly reliable method used for infants and young children for RSV diagnosis, however, shows poorer sensitivity in adults due to a lower rate of viral shedding compared to that in younger children.
Polymerase Chain Reaction
PCR detects the RSV in nasopharyngeal swabs and aspirates with high sensitivity and specificity. It is particularly useful for older children and adults and is preferred for hospitalized and immunocompromised patients. However, it is more expensive than DFA and may have a longer turnaround time in certain laboratory settings.
Viral Culture
Viral culture is not recommended for initial clinical management due to a slow turnaround time of about 3-5 days with lower sensitivity. However, it is still important for the detection of coinfections. It has high specificity and the virus can be stored for diagnostic studies as well.
Other laboratory tests to diagnose RSV complications include:
- Blood tests for examination of white cell counts or detection of viruses, bacteria, and other germs
- Chest X-rays to check for lung inflammation
- Pulse oximetry (that uses a painless skin monitor) for the detection of oxygen levels lower than normal in the blood
Treatment of RSV
Mild infections usually do not require any treatment and antibiotics or bronchodilators are not used during RSV infections.
Supportive treatment
- Acetaminophen (Tylenol and other medications) may be given to the patient to reduce fever. However, aspirin should never be given to a child.
- Nasal saline drops and suctioning can be used to help clear a stuffy nose.
- Antibiotics can be prescribed in case of bacterial infection, such as bacterial pneumonia.
- It is important to drink enough fluids to prevent dehydration and also look out for signs of dehydration like dry mouth, little to no urine output, sunken eyes, and extreme fussiness or sleepiness.
Hospital treatment
For further complications, treatments at the hospital may include:
- Intravenous (IV) fluids
- Humidified oxygen
- A breathing machine (mechanical ventilation in extreme cases)
Currently, there are no vaccines licensed and used for RSV infections but a number of vaccine candidates are in their clinical trial phases testing the safety and efficacies of these vaccines. However, palivizumab can be administered to infants and young children who are at high risk for severe disease.
Prevention and Control of RSV
As RSV is highly contagious, suitable preventive interventions must be taken to prevent its spread. Some of the measures include:
- Avoiding close contact with infected people
- As the virus can be transmitted through inanimate objects, sharing cups, bottles, towels, toys, utensils, etc. that have been contaminated with the virus must be avoided
- Thoroughly washing hands with soap and water after coming in contact with an infected individual
- Covering the mouth while sneezing or coughing with a tissue or upper shirt sleeve and not with the hand
- People with flu-like symptoms should avoid contact with children at high risk for severe RSV diseases, including premature infants, children younger than 2 years of age, children with chronic lung or heart conditions, and children with a weakened immune system.
- Educating parents, caregivers, and health care providers about the risk, transmission, preventive measures, and other factors associated with RSV infections
- Passive immunizations like RSV-IGIV and palivizumab have been developed for RSV infections. However, the polyclonal immunoglobulin product from the pooled blood donors; RSV-IGIV, is no longer available.
References
- Taleb, S.A., Al Thani, A.A., Al Ansari, K. et al. Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches. Eur J Clin Microbiol Infect Dis 37, 1817–1827 (2018).
- Godefroy, R., Giraud-gatineau, A., Jimeno, M.-T., Edouard, S., Meddeb, L., Zandotti, C., Chaudet, H., Colson, P., Raoult, D., & Cassir, N. (2020). Respiratory Syncytial Virus Infection: Its Propensity for Bacterial Coinfection and Related Mortality in Elderly Adults. Open Forum Infectious Diseases, 7(12). https://doi.org/10.1093/ofid/ofaa546
- Mammas, I. N., Drysdale, S. B., Rath, B., Theodoridou, M., Papaioannou, G., Papatheodoropoulou, A., koutsounaki, E., Koutsaftiki, C., Kozanidou, E., Achtsidis, V., Korovessi, P., Chrousos, G. P., & Spandidos, D. A. (2020). Update on current views and advances on RSV infection (Review). International Journal of Molecular Medicine, 46(2), 509–520. https://doi.org/10.3892/ijmm.2020.4641
- Diagnosing and Treating RSV. (n.d.). Www.lung.org. Retrieved April 1, 2022, from https://www.lung.org/lung-health-diseases/lung-disease-lookup/rsv/treatment#:~:text=Avoiding%20close%20contact%20with%20infected
- RSV | Prevention | Respiratory Syncytial Virus | CDC. (2019, February 4). Www.cdc.gov. https://www.cdc.gov/rsv/about/prevention.html
- Samson, L. (2009). Prevention of respiratory syncytial virus infection. Paediatrics & Child Health, 14(8), 521–526. https://doi.org/10.1093/pch/14.8.521
- RSV. (2019). https://www.cdc.gov/rsv/about/symptoms.html
- Respiratory syncytial virus – Diagnosis and treatment – Mayo Clinic. (2021, January 9). Www.mayoclinic.org. https://www.mayoclinic.org/diseases-conditions/respiratory-syncytial-virus/diagnosis-treatment/drc-20353104
- Respiratory Syncytial Virus – RSV | ARUP Consult. (n.d.). Arupconsult.com. https://arupconsult.com/content/respiratory-syncytial-virus
- Mayo Clinic. (2017). Respiratory syncytial virus – Symptoms and causes. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/respiratory-syncytial-virus/symptoms-causes/syc-20353098
- Mammas, I. N., Drysdale, S. B., Rath, B., Theodoridou, M., Papaioannou, G., Papatheodoropoulou, A., koutsounaki, E., Koutsaftiki, C., Kozanidou, E., Achtsidis, V., Korovessi, P., Chrousos, G. P., & Spandidos, D. A. (2020). Update on current views and advances on RSV infection (Review). International Journal of Molecular Medicine, 46(2), 509–520. https://doi.org/10.3892/ijmm.2020.4641
- Respiratory Syncytial Virus Infections. (2019). Medlineplus.gov; National Library of Medicine. https://medlineplus.gov/respiratorysyncytialvirusinfections.html
- VisualDx Cookie Check. (n.d.). Www.visualdx.com. Retrieved April 1, 2022, from https://www.visualdx.com/visualdx/diagnosis/respiratory+syncytial+virus+infection?diagnosisId=54823&moduleId=101
- Kiss, G., Holl, J. M., Williams, G. M., Alonas, E., Vanover, D., Lifland, A. W., Gudheti, M., Guerrero-Ferreira, R. C., Nair, V., Yi, H., Graham, B. S., Santangelo, P. J., & Wright, E. R. (2014). Structural Analysis of Respiratory Syncytial Virus Reveals the Position of M2-1 between the Matrix Protein and the Ribonucleoprotein Complex. Journal of Virology, 88(13), 7602–7617. https://doi.org/10.1128/JVI.00256-14
- Collins, P. L., Fearns, R., & Graham, B. S. (2013). Respiratory Syncytial Virus: Virology, Reverse Genetics, and Pathogenesis of Disease. Current Topics in Microbiology and Immunology, 372, 3–38. https://doi.org/10.1007/978-3-642-38919-1_1
- Schweitzer, J. W., & Justice, N. A. (2018, October 27). Respiratory Syncytial Virus Infection (RSV). Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459215/

Hi, my name is Francisco and I’m a 6th year medicine student. I´ve seen this publication and I liked a lot the RSV structure illustration. I wonder if I could use it for my Final degree project.
Thanks for your attention.
Hi Francisco.
Thank for liking our RSV structure illustration. Sure you can use the figure with citation to our website.