Post-translational modifications refer to any alteration in the amino acid sequence of the protein after its synthesis.
- It may involve modifying the amino acid side chain, terminal amino or carboxyl group using covalent or enzymatic means following protein biosynthesis.
- Generally, these modifications influence the structure, stability, activity, cellular localization, or substrate specificity of the protein.
- The post-translational modification provides complexity to the proteome for diverse functions with a limited number of genes.
Location
Post-translational modifications (PTMs) mainly occur in the endoplasmic reticulum of the cell but sometimes continue in the Golgi bodies as well.
Interesting Science Videos
Post-translational Processing
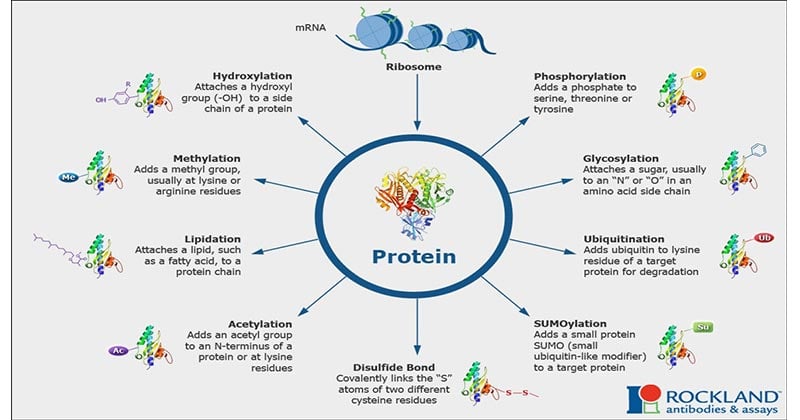
After synthesis is completed, proteins can be modified by various methods such as phosphorylation, glycosylation, ADP ribosylation, hydroxylation, and addition of other groups.
1. Proteolysis
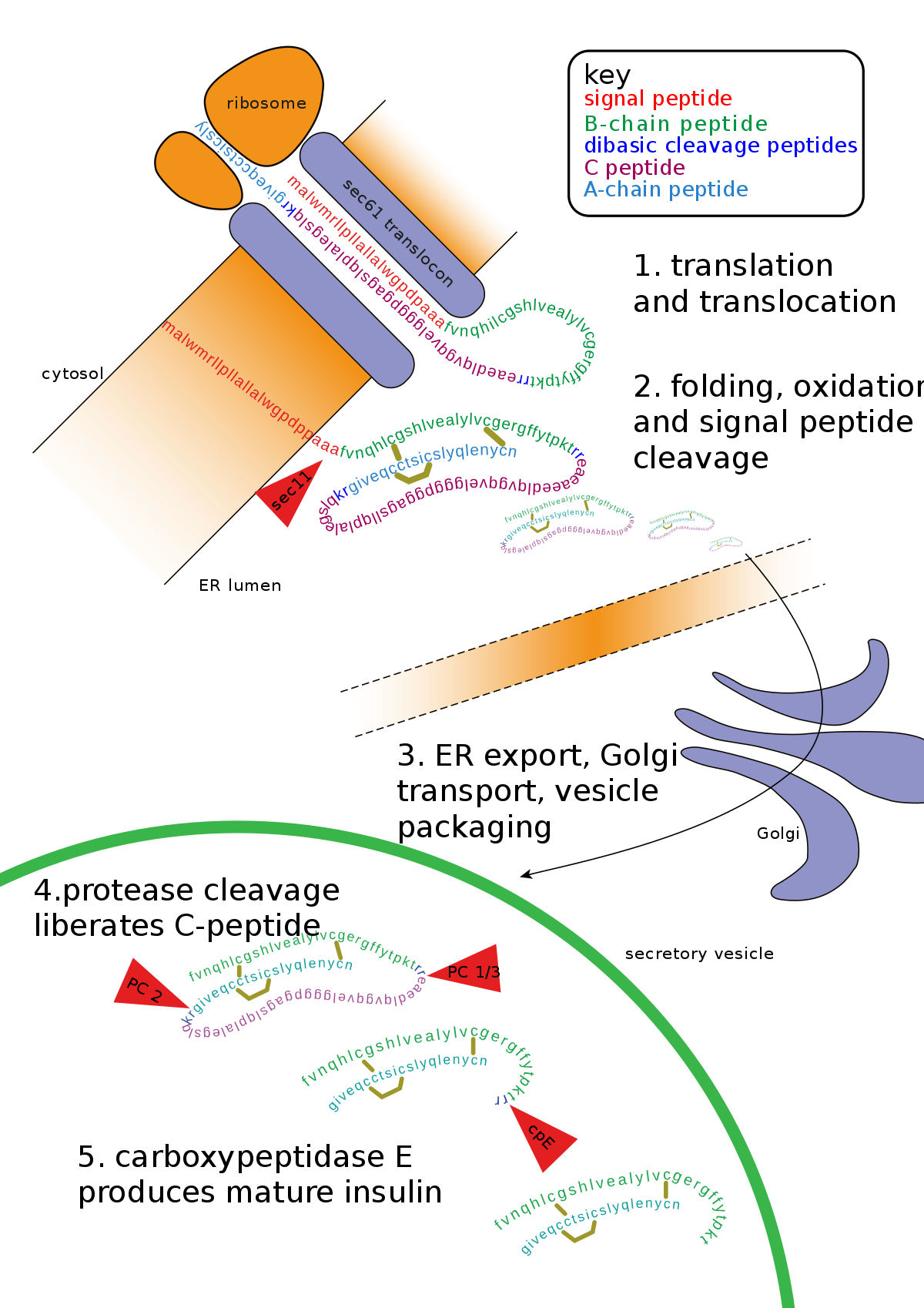
As the newly synthesized protein is released in the lumen of the ER, signal peptidases cleave peptide sequence. Apart from signal peptide, some polypeptide sequence of the protein is also cleaved resulting in the final sequence.
Example:
Insulin is synthesized in the cells in its inactive form which cannot perform its function. Post translational modifications ensure proper function which involves the removal of the part of protein to convert it into a three dimensional and fully active form.
2. Phosphorylation
Phosphoryalation is the addition of one or more phosphate groups to the protein. Post Translational Phosphorylation is one of the most common protein modifications that occur in animal cells. Majority of phosphorylation occurs as a mechanism to regulate the biological activity of a protein. In animal cells Serine, tyrosine and thereonine are the amino acids that subjected to the phosphorylation.
3. Glycosylation
Glycosylation is the addition of carbohydrate molecules to the polypeptide chain and modifying it into glycoproteins. Many of the proteins that are destined to become a part of plasma membrane or to be secreted from the cell, have carbohydrate chains attached to the amide nitrogen of asparagine(N linked) or the hydroxyl groups of serine, threonine(O linked). N glycosylation occurs in ER and O glycosylation occurs in the golgi complex.
4. Sulfation
Sulfate modidication takes place by the addition of sulphate molecules and these modifications of proteins occurs at tyrosine residues. Tyrosine sulfation accomplished via the activity of tyrosylproteinsulfotransferases (TPST) which are membrane associated enzymes of trans-Golgi network. There are two known TPSTs. TPST-1 TPST-2 The universal phosphate donor is 3’-phosphoadenosyl- 5’-phosphosulphate (PSPA).
5. Methylation
The transfer of one-carbon methyl groups to nitrogen or oxygen to amino acid side chains increases the hydrophobicity of the protein and can neutralize a negative amino acid charge when bound to carboxylic acids. Methylation is mediated by methyltransferases and S-adenosyl methionine (SAM) is the primary methyl group donor.
6. Hydroxylation
The biological process of addition of a hydroxy group to a protein amino acid is called Hydroxylation. Protein hydroxylation is one type of PTM that involves the conversion of –CH group into –COH group and these hydroxylated amino acids are involved in the regulation of some important factors called transcription factors. Among the 20 amino acids, the two amino acids regulated by this method are proline and lysine.
7. Others
a) SUMOylation
SUMO (small ubiquitin related modifier) proteins are 100 amino acid residue proteins which bind to the target protein in the same way as ubiquitin. They also confer the transcription regulatory activity of the protein and help in the transport of the target protein from cytosol to the nucleus.
b) Disulfide bond formation
Stabilizes protein structure and involved in redox processes.
c) Lipidylation, Acetylation, Prenylation etc.
After synthesis is completed, proteins can be modified by various methods such as phosphorylation, glycosylation, ADP ribosylation, hydroxylation, and addition of other groups.
Significance
Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo modifications to form the mature protein product.
Post-translational modifications of proteins, which are not gene- template based, can regulate the protein functions, by causing changes in protein activity, their cellular locations and dynamic interactions with other proteins.
PTMs have significant biological functions which include:
- Aids in proper protein folding – few lectin molecules called calnexin binds to glycosylated proteins and assist in its folding.
- Confers stability to the protein- glycosylation can modify the stability of the protein by increasing protein half life.
- It protects the protein against cleavage by proteolytic enzyme by blocking the cleavage sites.
- Protein sorting or translocation- If phosphorylated mannose residues are present in the protein it always goes to lysosome.
- It regulates protein activity and function- phosphorylation of protein is a reversible PTM which activates the protein.
- Acetylation regulates many diverse functions, including DNA recognition, protein-protein interaction and protein stability.
- Redox-dependent PTM of proteins is emerging as a key signaling system conserved through evolution, influences many aspects of cellular homeostasis.
- PTMs are important components in cell signaling, as for example when prohormones are converted to hormones.
- It significantly increases the diversity and complexity in the proteome.
References
- https://nptel.ac.in/courses/102103017/pdf/lecture%2019.pdf
- https://en.wikipedia.org/wiki/Post-translational_modification
- https://www.slideshare.net/RIZWANABBAS3/post-translational-modification-80774583
- David Hames and Nigel Hooper (2005). Biochemistry. Third ed. Taylor & Francis Group: New York.
- Madigan, M. T., Martinko, J. M., Bender, K. S., Buckley, D. H., & Stahl, D. A. (2015). Brock biology of microorganisms (Fourteenth edition.). Boston: Pearson.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.

Very good write-up. I certainly love this website. Stick with it!|