Interesting Science Videos
What is positive staining?
Positive staining of viruses is similar to negative staining. The difference is that the virus image is darker formed on a light background, unlike the negative staining where a light viral particle image is formed on a dark background. The technique is simple and it is used widely to study the diverse morphologies of viruses from aquatic environments.
Principle for positive staining of viruses
- This procedure is light sensitive and therefore should be performed in an environment that is protected from light.
- Similar to negative staining, the sample is incubated with a heavy metallic solution, which reacts with the cell organelles and structures.
- In positive staining, uranyl acetate and/or lead citrate are the most commonly used salts.
- Ultra-thin sections of the samples are incorporated into a grid and the uranyl acetate salt added.
- Washing which is a very critical process of the stain is done to eliminate excess salts from outside the stain.
- Airdried grids of the sample are viewed under the Transmission Electron Microscope.
Procedure of Positive Staining of Viruses
Materials and reagents
- 2-3 pairs of EM grid-grade tweezers
- Uranyl acetate
- Ultra-purified water
- Petri plate
- 4 pieces of 2ml microcentrifuge tubes with screw caps
- 4ml plastic culture tube with cap
- stir bar
- stir plate
- Filter paper (cut into wedges)
- 0.02 µm syringe filter and 3-5 ml syringe
- Lab coat, respiratory protection (mask), eye protection
- Timer
- waste container for uracyl acetate
Preparation of 2% Uranyl acetate
- 0.04 gm uranyl acetate + 2 ml Q-water = 2% uranyl acetate
Procedure using uranyl acetate
- Prepare 2ml of 2% uranyl acetate with ultra-purified water in a 4ml culture tube and stir with a stir bar to mix the sample on a stir plate for approximately 30 minutes to 1 hour.
- Filter the uranyl acetate solution using a 0.02 µm syringe filter into a 2 ml screw-cap tube to remove any undissolved particles.
- Fill the 2 ml screw-cap tube with ultra-purified water
- Immerse the grid into the 25 uranyl acetate solution for 30 seconds
- Immerse the grid into the 1 tube of ultra-purified water for 10 seconds
- Immerse the grid into the 2nd tube of ultra-purified water for 10 seconds
- Immerse the grid into the 3rd tube of ultra-purified water for 10 seconds
- Wick off liquid from the grid using a wedge of filter paper placed at the edge of the grid.
- Place the grid in a grid box and allow it to dry overnight.
- Observe under a Transmission electron Microscope.
Procedure using Uranyl acetate and lead citrate
This procedure should be performed in an environment protected from light and free of CO2.
- The ultra-thin section of the sample is added to the grid.
- The grid with the sample is incubated in uranyl acetate for 15 minutes.
- Wash the grids in ultra-purified water
- Incubate the grids in lead citrate for 4-5 minutes in a CO2 free environment, by placing NaOH tablets during the experiment to absorb and CO2 that may react with the salts.
- The grids are then airdried and observed under the Transmission microscope.
Results and interpretation
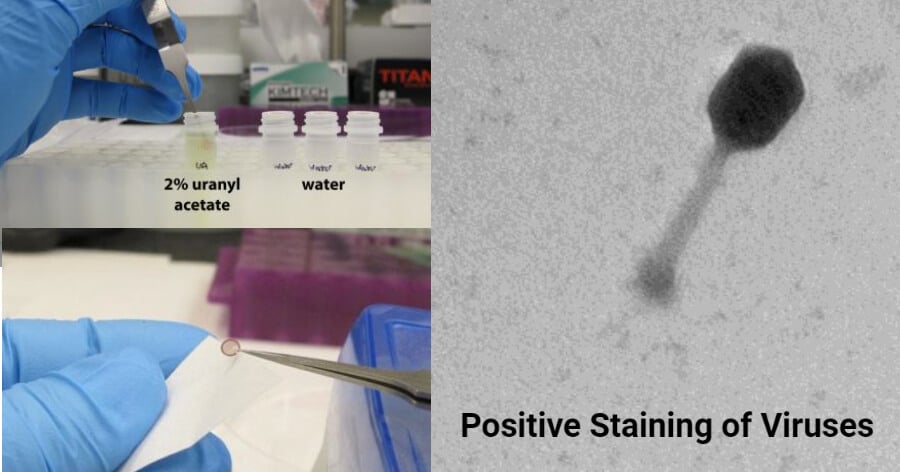
- Positive staining under a Transmission Electron Microscope (TEM) shows a dark image of the virus and a light background, showing the physical morphology of the virus, revealing any structural elements such as pikes and envelope of the virus.
- For example, the use of uranyl acetate to study Hepatitis B antigen showed the spread of viral particles all over the grid.
Advantages of Positive staining
- It is simple to perform.
- It is rapid to perform.
Limitations of Positive staining of viruses
- External reaction with CO2 may affect the result and orientation of the morphology of the viruses.
- Exposure to small light rays may affect the outcome of the results.
Applications of Positive Staining of Viruses
- It has been used to visualize various morphologies of viruses such as viral spikes, and envelopes, for viruses such as orthomyxoviruses, adenoviruses, hepatitis, rhinoviruses, influenza viruses.
- It is used to study the morphologies and physiological features of viruses.
- It has been used to identify and differentiate various viruses such as animal viruses from human viruses.
Progress in Positive staining of viruses
- New techniques are being developed to positively identify viruses using the Transmission electron Microscope to observe novel morphologies and physiological features of viruses.
- For example, the Tokuyasu staining procedure (TSP), is a positive staining method using Uranyl acetate, glutaraldehyde, and polyvinyl alcohol to identify and visualize non-enveloped and enveloped viruses.
- This includes rotaviruses, rubella viruses, HIV-1, Human T cell Lymphotropic virus.
- This stain has been used in identifying novel features of rotavirus and rubella viruses as well.
References and Sources
- 3% – https://microbenotes.com/negative-staining-of-viruses/
- 2% – https://www.sciencedirect.com/topics/materials-science/transmission-electron-microscopy
- 2% – https://www.researchgate.net/publication/291354740_Negative_and_Positive_Staining_in_Transmission_Electron_Microscopy_for_Virus_Diagnosis
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4789507/
- 1% – https://www.microscopemaster.com/transmission-electron-microscope.html
- 1% – https://en.m.wikipedia.org/wiki/Uranyl_acetate
- <1% – https://www.researchgate.net/post/Does_anybody_have_a_protocol_for_uranyl_acetate_staining_of_nanocellulose
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2978762/
- <1% – https://greenbioresearch.com/product/2ml-screw-cap-microcentrifuge-tubes-conical-bottom-freezer-micro-tubes/

More blessings good job tnx you