Interesting Science Videos
What is a peptide?
- A peptide is a short-chain made up of amino acid which, together with other peptides, forms a protein.
- The number of amino acids in a peptide can range from two amino acids to fifty amino acids.
- Based on the number of amino acids present in the peptide, peptides are of many types; peptides with ten or fewer amino acids are termed oligopeptides, and the peptides with more than ten amino acids are termed polypeptides.
- Polypeptides with around 100 amino acids are then considered proteins.
Peptide bond definition
- A peptide bond is a special type of amide bond formed between two molecules where an α-carboxyl group of one molecule reacts with the α-amino group of another molecule releasing a water molecule.
- The peptide bond is also referred to as the isopeptide bond where the amide bond forms between the carboxyl group of one amino acid and the amino group of another amino acid at other positions than the alpha.
- The process of formation of the peptide bond is an example of a condensation reaction resulting in dehydration (removal of water).
- Peptide bonds are covalent bonds that exist between any two amino acids resulting in a peptide chain.
- A partial double bond exists between carbon and nitrogen of the amide bond which stabilizes the peptide bond.
- The nitrogen involved in the bond donates its lone pair to the carbonyl group resulting in a resonance effect.
- The resonance is highly stabilizing as the electrons can be delocalized over multiple atoms resulting in a resonance structure.
- Thus, the resonance structure stabilizes the bond but also limits the rotation around the amide bond due to the partial double bond.
- Peptide bonds have a planar configuration that undergoes very little movement around the C-N bond but the other single bonds on either side of the C-N bond exhibit a high degree of rotational motion.
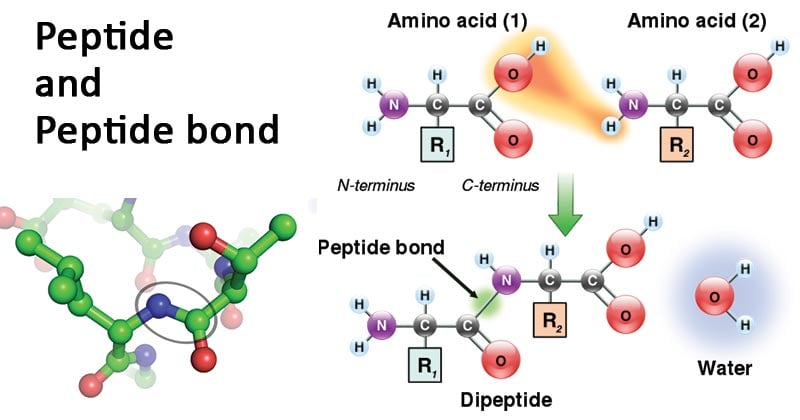
Image Source: Wikipedia.
Peptide bond formation mechanism
- The mechanism of peptide bond formation is a dehydration synthesis process.
- During the formation of a peptide bond, the carboxyl group of one amino acid moves towards the amino group of another amino acid.
- Subsequently, one hydrogen and one oxygen atoms are lost from the carboxyl group (COOH) of the first amino acid. In contrast, one hydrogen is lost from the amino group (NH2) of the other amino acid.
- This results in the release of a water molecule (H2O) along with the formation of an amide bond (C-N) between the two amino acids.
- The process of formation of a peptide bond between two amino acids results in a dipeptide molecule.
- Thus, a peptide bond is formed when the carboxyl group of one amino acid condenses with the amino group of another amino acid releasing in a water molecule.
- The formation of the peptide bond is an endergonic reaction that requires energy, which is obtained from ATP in living beings.
- Because this reaction involves the removal of a water molecule, it is called a dehydration synthesis reaction.
Peptide bond degradation mechanism
- The degradation of the peptide bond takes place through hydrolysis, thus requires the presence of water molecules.
- The degradation reaction is very slow as the amide bond between the amino acids is stabilized by the partial double bond.
- Because of the partial double bond between carbon and nitrogen molecule, carbon atom generates a slight positive charge.
- In the presence of water, the OH– ions of water attack the carbon atom, which results in degradation of the peptide bond.
- The remaining hydrogen ion of the water then attacks the nitrogen atom resulting in the amino group.
- As a result of this, the peptide molecule is cleaved into two units; one unit with the carboxyl group and another with the amino group.
- The degradation of the peptide is an exergonic reaction that releases about 8-16 Kjol/mole of energy.
- Because the protein degradation reactions are very slow, they are usually catalyzed by proteolytic enzymes like proteases and peptidases.
Peptide bond hydrolysis
- Peptide bond hydrolysis is the primary step of all protein hydrolysis reactions.
- The most common method of protein degradation is acid-catalyzed hydrolysis of the peptide bond.
- Peptide hydrolysis is also essential in some synthetic reactions where amino acids in one peptide are cleaved and transferred to another peptide, resulting in separate peptide synthesis.
- Similarly, different peptides and proteins accumulate in cells resulting in toxicity. Peptide bond hydrolysis is essential in the removal of those toxins as well.
- Peptide bond hydrolysis is also an important step in the digestion of proteins in living beings.
- Hydrolysis of peptide bond occurs in the presence of water and is catalyzed by the presence of acid.
- Peptide bond hydrolysis is one of the mechanisms of peptide bond degradation where polypeptides are either cleaved into smaller peptides, or smaller peptides are degraded into separate amino acids.
Examples
- The peptide bond is present in all proteins that bind the amino acid in the chain together.
Monopeptide: having one amino acid
Dipeptide: having two amino acids
Tripeptide: having three amino acids
Tetrapeptide: having four amino acids
Pentapeptide: having five amino acids
Hexapeptide: having six amino acids
Heptapeptide: having seven amino acids
Octapeptide: having eight amino acids
Peptide bond formation video animation (Khan Academy)

Revision Questions/FAQs
What is a peptide bond?
A peptide bond is a special type of amide bond formed between two molecules where an α-carboxyl group of one molecule reacts with the α-amino group of another molecule releasing a water molecule.
Which parts of amino acids are involved in a peptide bond?
The carboxyl group of one amino acid and the amino group of another amino acid are involved in a peptide bond.
How do you identify a peptide bond?
Biuret test can be used to identify a peptide bond.
Is a peptide bond covalent?
Yes, a peptide bond is a covalent bond.
References
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition.
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company.
- ARLINGHAUS R, SHAEFER J, SCHWEET R. MECHANISM OF PEPTIDE BOND FORMATION IN POLYPEPTIDE SYNTHESIS. Proc Natl Acad Sci U S A. 1964; 51(6):1291-1299. DOI:10.1073/pnas.51.6.1291
Sources
- 4% – https://www.peptidesciences.com/information/peptide-bonds/
- 2% – https://quizlet.com/164305942/study-flash-cards/
- 2% – https://quizlet.com/116549295/chapter-4-bio-302-flash-cards/
- 1% – https://www.sciencedaily.com/terms/peptide_bond.htm
- 1% – https://www.ncbi.nlm.nih.gov/books/NBK21750/
- 1% – https://encyclopedia2.thefreedictionary.com/Polypeptide+chains
- 1% – https://en.wikipedia.org/wiki/Peptide_bond
- 1% – https://en.wikipedia.org/wiki/Isopeptide_bond
- 1% – https://en.m.wikipedia.org/wiki/Peptide_bond
- 1% – https://answersdrive.com/what-is-a-chain-of-peptides-1326184
- <1% – https://www.researchgate.net/publication/6976681_Resonance_Structures_of_the_Amide_Bond_The_Advantages_of_Planarity
- <1% – https://www.researchgate.net/publication/6835267_Opinion_-_Do_we_underestimate_the_importance_of_water_in_cell_biology
- <1% – https://www.chegg.com/homework-help/questions-and-answers/1-bond-carbon-nitrogen-considered-polar-covalent-atom-c-n-bond-develop-partial-negative-ch-q34037077
- <1% – https://chem.libretexts.org/Courses/University_of_Illinois%2C_Springfield/UIS%3A_CHE_124_(Morsch_and_Andrews)/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/18%3A_Amino_Acids%2C_Proteins%2C_and_Enzymes/18.03_Peptides
- <1% – https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/15%3A_Organic_Acids_and_Bases_and_Some_of_Their_Derivatives/15.15%3A_Formation_of_Amides
