Novobiocin is a member of the aminocoumarin antibiotic class produced by Streptomyces niveus Actinobacteria which inhibit the bacterial DNA synthesis process. Novobiocin binds with the GyrB subunit of the DNA gyrase enzyme and inhibits the DNA transcription process. It is also found to inhibit the action of DNA topoisomerase IV. It was initially proposed as an antibiotic to treat the infections caused by Gram-positive cocci. In September 1964, it was approved as an indicated treatment option to treat the infections of Staphylococcus aureus if other antibiotics were resistant; however, due to its toxicity and lower effectiveness, in 2009 the FDA put a ban on its use as a treatment measure.
Although novobiocin is not used for treatment, it is still used in clinical laboratories in the Novobiocin Susceptibility Test for the identification and differentiation of Staphylococcus spp. In 1975, Kloos and Schleifer found that S. aureus and most of the coagulase-negative Staphylococci spp. reported as pathogens are sensitive to novobiocin, but S. saprophyticus is innately resistant to it. They used this concept and devised a novobiocin susceptibility test to differentiate S. saprophyticus from other CONS.
Interesting Science Videos
Objective
- Primarily, to differentiate Staphylococcus saprophyticus from other Coagulase Negative Staphylococci (CONS), especially Staphylococcus epidermidis.
- Differentiation of CONS into two groups; novobiocin susceptible CONS and novobiocin resistant CONS.
Principle of Novobiocin Susceptibility Test
Novobiocin interferes with the bacterial DNA synthesis process; hence inhibits bacterial replication and colony formation. Many of the Staphylococcus species, both CONS and Coagulase Positive Staphylococci, are susceptible to novobiocin. Among the pathogenic members of Staphylococcaceae, S. aureus, S. saprophyticus, S. epidermidis, S, hominis, and S. haemolyticus are the most common. S. saprophyticus and S. epidermidis are the most common uropathogens in the CONS group. All these pathogens are sensitive to novobiocin except S. saprophyticus. This antimicrobial sensitivity pattern is the principle based on which we can easily differentiate S. saprophyticus among other common CONS.
Requirements
Culture Media
Generally, Mueller Hinton Agar or Blood Agar is preferred for conducting the novobiocin susceptibility test. However, other general cultural media can also be used.
Preparation of MHA Plate
- Measure the appropriate amount of MHA powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company.
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve completely in water.
- Autoclave the flask or bottle at 1210C and 15 lbs pressure for 15 minutes and let it cool to around 40 – 450C.
- In a sterile Petri plate (glass plate with 10 cm diameter), pour around 25 mL of the MHA (make around 4 mm thickness of the media in the petri plate).
- Let the media solidify completely by leaving it at room temperature. (Store in a freeze at 40C for use up to 4 weeks)
Preparation of BA Plate (BAP)
- Measure the appropriate amount of Blood agar base powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company.
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve completely in water.
- Autoclave the flask or bottle at 1210C and 15 lbs pressure for 15 minutes and let it cool to around 40 – 450C.
- Pour 5% (5 to 10%) v/v sterile defibrinated blood (defibrinated Sheep Blood is preferred) in the flask with blood agar base slowly with constant stirring. Mix properly so that blood dissolves uniformly in the medium. This mixture is the Blood Agar.
- In a sterile Petri plate (glass plate with 10 cm diameter), pour around 25 mL of the blood agar and let it solidify properly by leaving it at room temperature. (Store BAPs in a freeze at 40C for use up to 2 to 4 weeks maximum)
Reagents
- Novobiocin Antibiotic Disk (5 μg)
- Defibrinated Sheep Blood (for BAP preparation)
- McFarland Standard (1 McFarland and 0.5 McFarland suspension)
Composition
1% anhydrous barium chloride (BaCl2) solution
1% sulfuric acid (H2SO4) solution
Preparation of number 1 McFarland Standard Suspension
- In a clean and clear test tube, add 9.9 mL of 1% H2SO4 and 0.1 mL of 1% BaCl2 solution
Preparation of number 0.5 McFarland Standard Suspension
- In a clean and clear test tube, add 9.95 mL of 1% H2SO4 and 0.05 mL of 1% BaCl2 solution
Equipment
| Petri Plates Forceps | Weighing Machine Autoclave | Bunsen burner Sterile Test Tubes | Micropipette Inoculating loop (Cotton Swab) |
PPE and other general laboratory materials.
Test bacteria
- Gram-positive, catalase-positive cocci in a cluster or short chain (Staphylococcus spp.))
- Staphylococcus aureus ATCC 25923
- Staphylococcus saprophyticus ATCC 15305
Procedure of Novobiocin Susceptibility Test
In general, this test can be performed using two slightly different methods; the Hebert method using BAP, and Kirby Bauer Disk Diffusion Method using an MHA plate.
- If you are using BAP, make a test bacterial suspension with sterile distilled water (or tryptic soya broth) in a sterile test tube with turbidity equal to McFarland number 1.
If you are using MHA, make bacterial suspension with sterile distilled water (or tryptic soya broth) in a sterile test tube with turbidity equal to McFarland number 0.5. - Using either a cotton swab or inoculating loop, inoculate the bacterial suspension in agar plates.
*In BAP, inoculate by streaking/spreading one section of the plate in one direction.
*In MHA, inoculate by streaking/spreading in three directions (uniformly all over the plate) - Let the suspension to adhere the plate and dry, leaving the plate in an upright position for about 5 to 10 minutes.
- Using sterile forceps, place a novobiocin antibiotic disk in the area of inoculation and gently press the antibiotic disk to ensure its adherence.
- Incubate the inoculated plate aerobically at 35±2°C for overnight (18 hours for MHA and 24 hours for BAP).
- Following the incubation period, observe the formation of a zone of inhibition around the novobiocin antibiotic disk and measure the zone diameter.
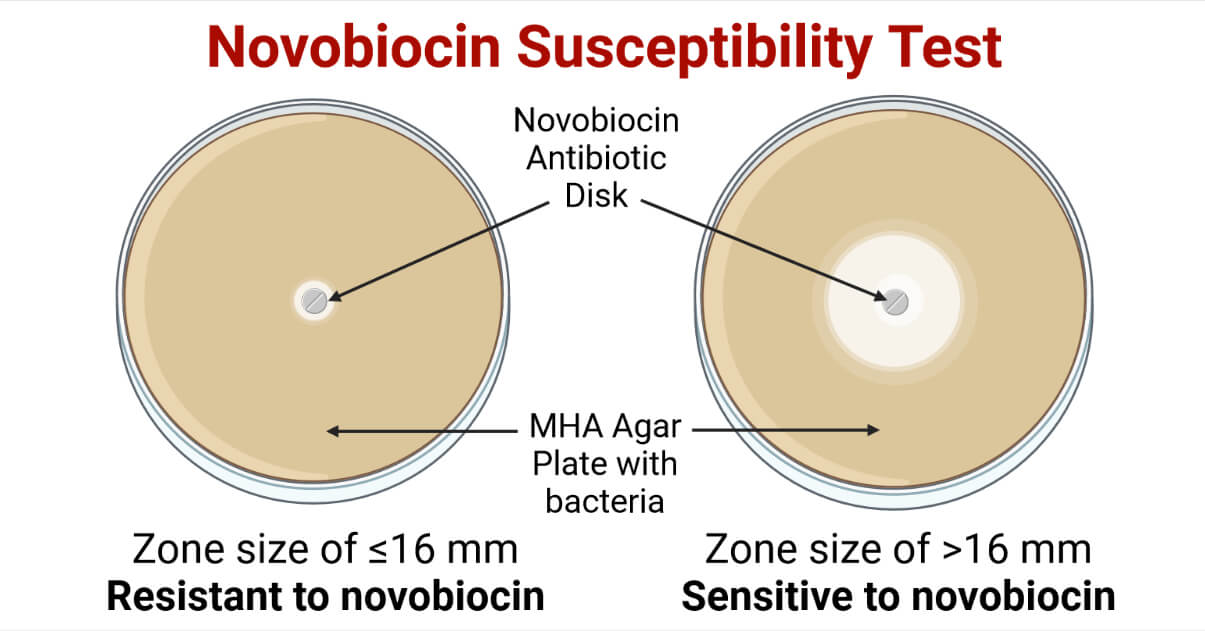
Result and Interpretation of Novobiocin Susceptibility Test
For BAP (Hebert method)
- Zone size of ≥12 mm = Sensitive to novobiocin
- Zone size of <12 mm = Resistant to novobiocin
For MHA (Kirby Bauer Disk Diffusion Method) (According to CLSI)
- Zone size of >16 mm = Sensitive to novobiocin
- Zone size of ≤16 mm = Resistant to novobiocin
If the test Staphylococcus spp. is isolated from a urine sample and it is coagulase-negative and novobiocin resistant, report it as Staphylococcus saprophyticus.
(However, it doesn’t confirm the bacterium as S. saprophyticus and must not be used if the bacteria are isolated from any samples other than urine.)
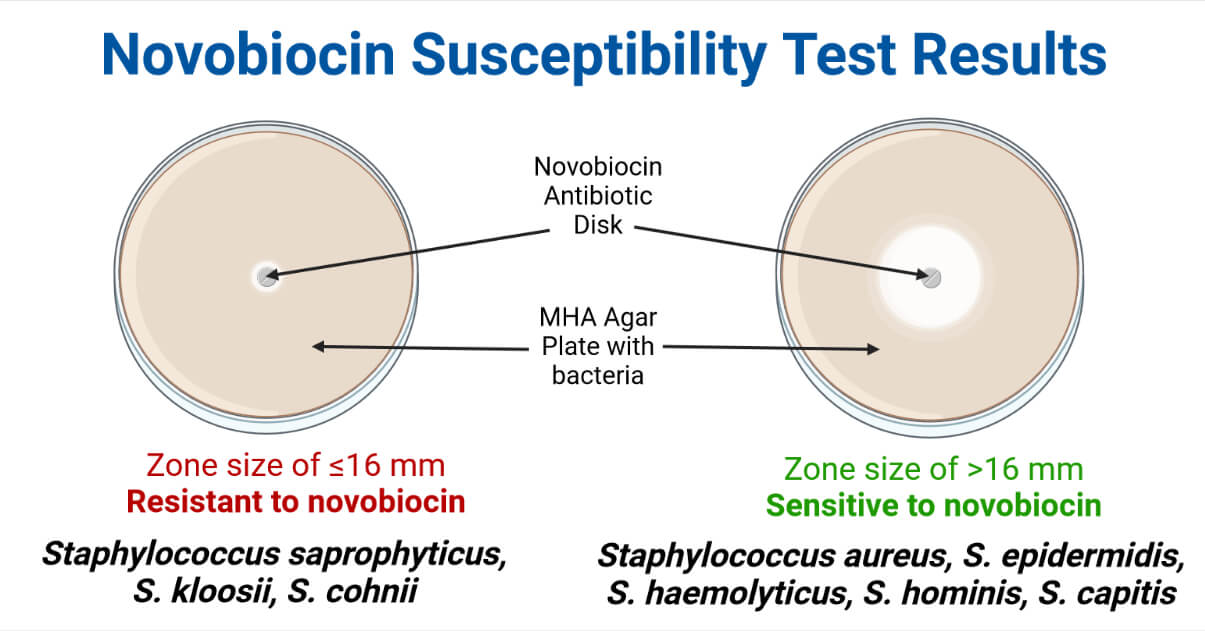
Quality Control
S. aureus ATCC 25923 (sensitive to novobiocin) is used as positive control where it develops antibiotic resistant zone of ≥22 mm around the novobiocin disk.
S. saprophyticus ATCC 15305 (resistant to novobiocin) is used as a negative control where it develops antibiotic resistant zone of ≤15 mm around the novobiocin disk.
Novobiocin Susceptible (Sensitive) Staphylococci
S. aureus, S. epidermidis, S. haemolyticus, S. hominis, S. capitis, S. saccharolyticus, etc.
Novobiocin Resistant Staphylococci
S. saprophyticus, S. kloosii, S. cohnii, etc.
Precautions
- Sterilize the medium properly before use, sterilize the working area, work in a sterile zone, wear proper PPE, and follow laboratory safety rules.
- Don’t pour blood in molten blood agar base if it is above 45°C.
- Pour the media into petri plates in a sterile zone before the temperature of the molten media drops below 40°C otherwise clumps may form.
- Ensure no air bubbles are formed while pouring the media. While leaving to solidify, place the plates in a sterile zone (inside the bio-safety cabinet) and leave the lid of the plate slightly open so that vapor can escape and there are no water drops over the solidified medium.
- Completely solidify the agar plates before use. Prepare it at least 6 hours (or 1 day) before use to ensure complete solidification. Never solidify media by placing it in the refrigerator, allow it to solidify at room temperature.
- Place the disk about 25 mm away from the edge of the plate. If using multiple antibiotic disks, place the disk at least 25 mm apart from each other.
Applications of Novobiocin Susceptibility Test
- For identification of S. saprophyticus among other pathogenic CONS.
- Differentiating S. epidermidis and S. saprophyticus during urine culture.
Limitations of Novobiocin Susceptibility Test
- It can be used as a confirmatory method to identify S. saprophyticus only if the bacteria are isolated from a urine sample.
- Need other biochemical tests to identify the bacterium.
- Since it is a culture-based method, it requires a longer duration and different chemicals/requirements.
- High chance of a false positive or false negative result.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 54675769, Novobiocin. Retrieved February 3, 2023 from https://pubchem.ncbi.nlm.nih.gov/compound/Novobiocin.
- Afanas’eva TI, Shcherbakova NA, Givental’ NI, Bogdanova LF. Znachenie opredeleniia chuvstvitel’nosti k novobiotsinu v taksonomii stafilokokkov [Significance of determining novobiocin sensitivity in the taxonomy of staphylococci]. Antibiotiki. 1979 Nov;24(11):824-7. Russian. PMID: 507776.
- De Paulis AN, Predari SC, Chazarreta CD, Santoianni JE. Five-test simple scheme for species-level identification of clinically significant coagulase-negative staphylococci. J Clin Microbiol. 2003 Mar;41(3):1219-24. doi: 10.1128/JCM.41.3.1219-1224.2003. PMID: 12624054; PMCID: PMC150284.
- Pereira, E.M., de Mattos, C.S., dos Santos, O.C. et al. Staphylococcus hominis subspecies can be identified by SDS-PAGE or MALDI-TOF MS profiles. Sci Rep 9, 11736 (2019). https://doi.org/10.1038/s41598-019-48248-4
- Bacitracin and Novobiocin Susceptibility Tests by Megan Lane (prezi.com)
- https://microbeonline.com/preparation-mcfarland-turbidity-standards/
- Novobiocin Susceptibility Test – Procedure, Uses and Interpretation (microbiologyinfo.com)
- Novobiocin Susceptibility Test Principle, Procedure, Results (microbiologynote.com)
- Novobiocin susceptibility test: Principle, Procedure and Results interpretations (onlinebiologynotes.com)
- Novobiocin susceptibility test – Virtual Microbiology Lab Simulator Software (vumicro.com)
- Novobiocin Susceptibility Test: Purpose, Procedure & Results (researchtweet.com)
- Gram-Positive Bacteria Identification: Introduction, List of Common (medicallabnotes.com)
