Interesting Science Videos
Nitrification Definition
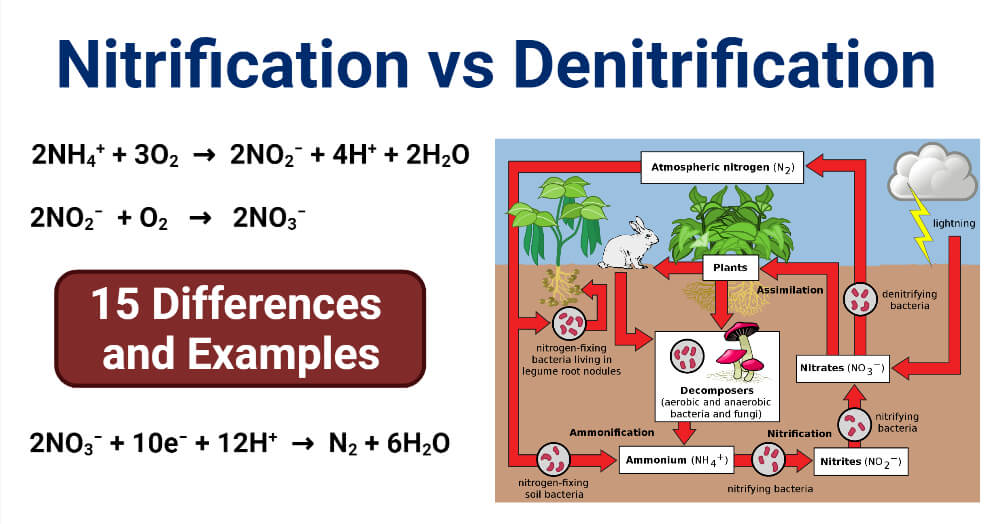
Nitrification is a biological process of oxidation of ammonia into nitrite which is then followed by the oxidation of nitrite into nitrate.
- Nitrification is a part of the nitrogen cycle where living beings oxidize ammonia present in the soil into useful forms of nitrogen that can then be consumed by various organisms.
- In the nitrogen cycle, nitrogen is converted into many forms successively passing from atmosphere to soil to organisms and back to the atmosphere.
- Nitrification is an aerobic process that occurs in soil by various aerobic microorganisms like bacteria and some archaea.
- The bacteria oxidizing ammonia are termed ammonia-oxidizing bacteria (AOB), and the archaea oxidizing archaea are termed ammonia-oxidizing archaea (AOA).
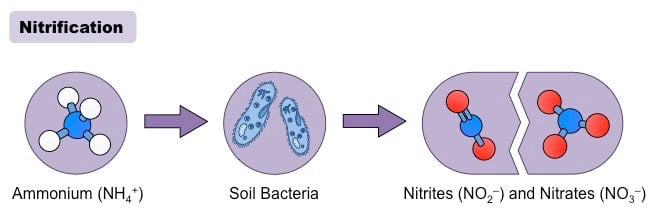
Image Source: BioNinja.
- The reactions involved in nitrification are the following:
2NH4+ + 3O2 → 2NO2– + 4H+ + 2H2O
2NO2– + O2 → 2NO3–
- Because this process consists of two reaction steps, two different groups of microorganisms are involved in nitrification.
- The first step of oxidation of ammonia is brought about by microbes in the soil which includes bacteria of the genus Nitrosomonas and Nitrosococcus, and arceae like Nitrosopumilus maritimus and Nitrososphaera viennensis. The conversion of ammonia into nitrite is the rate-limiting step of nitrification.
- The second reaction is performed by bacteria of the genus Nitrobacter and Nitrospira.
- All of these microorganisms are chemoautotrophs that utilize the energy from the reaction to produce organic carbon compounds.
- Nitrification is important in many organisms as it is the only process of obtaining nitrogen source for some microorganisms present in the soil.
- These organisms convert ammonia into nitrates which is more soluble than ammonia and thus can be taken into the system more conveniently.
- Besides, it is also important in agricultural systems where ammonia is used as a fertilizer. The ammonia is then converted into nitrate which facilitates nitrogen leaching into the plants.
- Nitrifying bacteria also play an important role in wastewater treatment where different nitrogen compounds are converted into nitrates and then nitrogen before removing the gas out of the water.
- The nitrification process is controlled by a number of factors like the availability of oxygen, soil moisture, and the availability of ammonia.
- The activity of the nitrifying bacteria also decreases in acidic conditions and at a temperature above 35°C.
Denitrification Definition
Denitrification is a biological process of reduction of nitrate into nitrite, which is then followed by the reduction of nitrate into nitrogen gas that usually results in the removal of nitrogen gas into the air.
- Denitrification, like nitrification, is a microbial process that is performed by various groups of microorganisms.
- It is also an important step in the nitrogen cycle where nitrogen is released back into the atmosphere from the ground.
- In this case, the oxidized products of nitrogen are reduced to its gaseous forms, mainly nitrous oxide (N2O) and nitrogen gas (N2).
- Denitrification, unlike nitrification, is performed by facultative anaerobes that perform denitrification as anaerobic respiration to reduce oxidized forms into gases.
- Denitrification takes place at about 10% or less concentration of oxygen and organic carbon compounds.
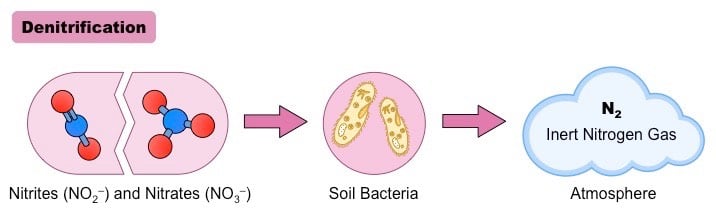
Image Source: BioNinja.
- The process of denitrification takes place through a set of half-reactions, which are:
NO3– + 2H+ + 2e– → NO2– + H2O
NO2− + 2 H+ + e− → NO + H2O
2NO + 2 H+ + 2 e− → N2O + H2O
N2O + 2 H+ + 2 e− → N2 + H2O
- The overall reaction can be represented as:
2NO3− + 10e− + 12H+ → N2 + 6H2O
- The process is primarily performed by heterotrophic bacteria like Paracoccus denitrificans and some species of Pseudomonas, but some autotrophic denitrifiers like Thiobacillus denitrificans are also present.
- Denitrification is an important microbiological process that is performed naturally in both terrestrial and marine environments.
- Besides, denitrification follows nitrification in wastewater treatments to convert nitrogen-rich compounds into nitrogen gas before being released into the atmosphere.
- However, sometimes denitrification can be disadvantageous by removing the NO3– present in the soil, thus reducing the extent of leaching.
- Denitrification is controlled by various factors like the concentration of oxygen and carbons, even though some aerobic bacteria of the genus Proteobacteria, might facilitate denitrification even in the presence of oxygen.
Key differences (Nitrification vs Denitrification)
| Basis for comparison | Nitrification | Denitrification |
| Definition | Nitrification is a biological process of oxidation of ammonia into nitrite which is then followed by the oxidation of nitrite into nitrate. | Denitrification is a biological process of reduction of nitrate into nitrite, which is then followed by the reduction of nitrite into nitrogen gas that usually results in the removal of nitrogen gas into the air. |
| Reaction | The overall reaction of nitrification is: NH4+ → NO2– → NO3– | The overall reaction of denitrification is: 2 NO3− + 10 e− + 12 H+ → N2 + 6 H2O |
| Steps | The process of nitrification consists of two reaction steps. | The process of denitrification occurs via a series of half-reactions. |
| Process | Nitrification is a process of oxidation reactions. | Denitrification is a process consisting of reduction reactions. |
| Nitrogen cycle | Nitrification is the second step of the nitrogen cycle. | Denitrification is the last step of the nitrogen cycle. |
| Involves | Nitrification involves the conversion of reduced nitrogen compounds into oxidized forms. | Denitrification involves the conversion of oxidized nitrogen compounds into reduced forms. |
| End product | The end product of nitrification is nitrate (NO3–). | The end product of denitrification is either nitrous oxide (NO2) or nitrogen gas (N2). |
| Substrate | The substrate or starting compound of the nitrification process is ammonia | The substrates or starting compounds of denitrification are nitrates and nitrites. |
| Oxygen concentration | Various microorganisms perform nitrification under a high concentration of oxygen. | Various microorganisms perform denitrification under a low concentration of oxygen. |
| Microorganisms involved | Common nitrifying microorganisms include Nitrosomonas, Nitrosococcus. Nitrobacter, Nitrospira, Nitrosopumilus maritimus, and Nitrososphaera viennensis. | Common denitrifying microorganisms include Paracoccus denitrificans, Thiobacillus denitrificans, Proteobacteria. |
| Nutrition | Most microbes involved in nitrification are chemoautotrophic. | Most microbes involved in nitrification are heterotrophic. |
| Required pH | Nitrification flourishes in the pH range of 6.5 to 8. | Denitrification flourishes in the pH range of 7 to 9. |
| Temperature | The process mostly occurs at a temperature of 20-30°C. | The process mostly occurs at a temperature of 26°C to 38°C. |
| Inhibitors | Some inhibitors associated with nitrification are sulfur-containing compounds and N-heterocyclic compounds. | Some inhibitors associated with denitrification are acetylene, cyanide, and some pesticides like Vapam. |
| Importance | Nitrification is an essential process as it helps to provide nitrates to plants which act as a source of nitrogen. | Denitrification is an important process that ensures the cyclic movement of nitrogen from the atmosphere to the soil, plants, and back to the atmosphere. Besides, it also plays an important role in wastewater treatments. |
Video: Nitrification & Denitrification
References and Sources
- Knowles R. (1982). Denitrification. Microbiological Reviews, 46(1), 43–70.
- 2% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3035804/
- 2% – https://pediaa.com/difference-between-nitrification-and-denitrification/
- 2% – https://infogalactic.com/info/Nitrification
- 1% – https://www.toppr.com/ask/question/nitrate-is-converted-into-ammonia-by-the-process-of/
- 1% – https://www.sciencedirect.com/topics/earth-and-planetary-sciences/aerobic-process
- 1% – https://www.researchgate.net/publication/313426362_RESEARCHES_IN_WATER_POLLUTION_A_REVIEW
- 1% – https://www.quora.com/What-chemicals-should-I-use-for-reaction-NO3-+-2e-+2H+-%E2%86%92-NO2-+-4-H2O
- 1% – https://www.greenandgrowing.org/nitrogen-cycle-what-when-where-why/
- 1% – https://en.wikipedia.org/wiki/Nitrification
- 1% – https://byjus.com/biology/nitrogen-cycle/
- <1% – https://en.wikipedia.org/wiki/Denitrifying_bacteria

