- Neisseria gonorrhoeae is an obligate human pathogen and is the etiological agent of gonorrhea.
- In the United States, it is the second most commonly reported communicable disease, with more than 350,000 cases reported annually.
- Syndromes include cervicitis in women and urethritis, pharyngitis and proctitis in both sexes. If untreated, women may experience severe sequelae of pelvic inflammatory disease, chronic pelvic pain, ectopic pregnancy, and tubal infertility, while men may develop epididymitis, prostatitis and urethral stricture.
Interesting Science Videos
Laboratory Diagnosis of Neisseria gonorrhoeae
A. Specimen choice and collection
- The specimen choice and collection method depends on the testing technique used in a laboratory and the age, sex and sexual orientation of the patient.
- Urethral: Express urethral exudates when patients have discharge. If there is no discharge, compress the meatus vertically to open the distal urethra and insert a thin, water-moistened swab (calcium alginate or Dacron) with flexible wire slowly (3 cm to 4 cm in males or 1 cm to 2 cm in females), rotate slowly and withdraw gently.
- Urine: Ask patients to collect only the first 10 mL to 15 mL of urine. Patients should not have voided for at least 2 h before specimen collection to increase the chance of detecting the organism.
- Cervical: Insert a speculum into the vagina to view the cervix. Insert a swab 1 cm to 3 cm into the endo-cervical canal and rotate for 10 s to 30 s to allow absorption of exudates.
- Vaginal: Collect pooled vaginal secretions, if present. Vaginal wash specimens are most preferred and acceptable to pre-pubertal girls. If not possible, rub a sterile cotton swab against the posterior vaginal wall and allow the swab to absorb the specimen.
- Rectal: Specimens may be obtained blindly or, preferably, through an anoscope. Insert a swab 2 cm to 3 cm into the anal canal. Avoiding fecal material, rotate to sample crypts just inside the anal ring; allow the swab to absorb specimen for 10s.
- Oropharyngeal: Rub sterile swabs over the posterior pharynx and tonsillar crypts, or obtain nasopharyngeal aspirate from infants.
- Conjunctiva: Any exudate or pus present in the eye should be carefully removed with a sterile swab. A second swab moistened with saline should be used to rub the affected conjunctiva. This swab should be broken off into a vial of transport medium.
- Sterile body fluids: Clean skin puncture site with iodine (1% to 2%, or 10% solution of povidone-iodine [1% free iodine]). If tincture of iodine is used, remove with 70% ethanol to avoid burn. Perform percutaneous aspiration for pleural, pericardial, peritoneal or synovial fluids. Use non-heparinized collection if possible.
B. Transport
- To minimize the inhibitory effects of unknown substances in the specimen, the swabs should be inoculated directly onto growth medium or placed in swab transport medium immediately after sampling.
- If the inoculated media are being transported to a local laboratory, the plates should be held at room temperature for no more than 5 h in a CO2-enriched atmosphere using a candle jar or a commercial CO2-generating system.
- If long-distance shipping is required, the specimens should be inoculated onto media contained in a CO2-generating system, incubated for 18 h to 24 h and have visible growth on the plate before shipping.
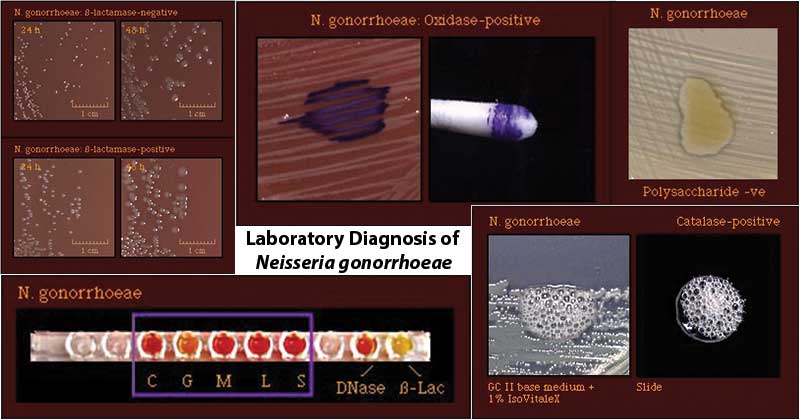
Image Source: CDC
C. Microscopy
- A direct smear for Gram staining may be performed as soon as the swab specimen is collected from the urethra, cervix, vagina or rectum.
- The Gram stain of a smear of urethral exudates or endocervical secretions shows typical Gram-negative, non-motile, intracellular diplococci.
D. Culture
- The current preferred laboratory method for the diagnosis of N. gonorrhoeae infections is the isolation and identification of the agent.
- The primary specimens should be inoculated onto nonselective chocolate agar and selective agar containing antimicrobial agents that inhibit the growth of commensal bacteria and fungi.
Growth medium:
- Modified Thayer Martin Medium(Chocolate agar containing antibiotics (vancomycin, colistin, trimethoprim, and nystatin)) is most often used.
- Modified Newyork City Medium (MNC)is also used for the culture of Neisseria gonorrhoeae.
- The inoculated plates should be incubated at 35°C to 37°C in a moist atmosphere enriched with CO2(3% to 7%).
- An 18 h to 24 h culture should be used as the inoculum for additional tests.
Note: Plates should not be incubated for longer than 48 h because most old cultures would not survive storage conditions. Autolysis may occur during prolonged incubation, and growth from agar plates becomes difficult to suspend in solutions.
- Isolates should be sub-cultured at least once on nonselective medium after initial isolation before being used in a diagnostic test that requires pure culture or heavy inoculum.
Presumptive identification:
- The presumptive identification of N. gonorrhoeae rests on the isolation of an oxidase-positive, catalase-positive, Gram-negative diplococcus recovered from urogenital sites that grow on selective media.
Confirmatory tests
- Confirmatory tests include biochemical tests, chromogenic enzyme substrate tests, immunoassays and nucleic acid methods.
Biochemical reactions:
- Oxidase Test: Positive
- Ferments glucose but not maltose, sucrose or lactose
- DNase Test: Negative
- Beta-galactosidase (ONPG) Test: Negative
- Glutamyl-aminopeptidase (GAP) Test: Negative
Enzyme substrate test:
- Hydroxyprolylaminopeptidase positive
Antimicrobial Susceptibility Testing
- Monitoring of antimicrobial susceptibilities of N. gonorrhoeae is important to investigate treatment failure and to evaluate the efficacy of currently recommended therapies.
- Antibiotic resistance in Neisseria gonorrhoeae has severely compromised the successful treatment of gonorrhea.
- Penicillins, tetracyclines, and newer macrolides have limited utility, and spectinomycin (and in many parts of the world, quinolones) have been withdrawn because of resistance.
- Of the usually recommended treatments, only the third-generation cephalosporins, and most notably ceftriaxone have retained their efficacy, but decreased susceptibility to these antibiotics has also appeared.
Treatment
- The current treatment recommended by the CDC is a dual antibiotic therapy.
- This includes an injected single dose of ceftriaxone (a third-generation cephalosporin) along with azithromycin administered orally. Azithromycin is preferred for additional coverage of gonorrhea that may be resistant to cephalosporin but susceptible to macrolides.
- Sexual partners (defined by the CDC as sexual contact within the past 60 days) should also be notified, tested, and treated.
- It is important that if symptoms persist after receiving treatment of N. gonorrhoeae infection, a reevaluation should be pursued.
Prevention of Infection
- Transmission can be reduced by using latex barriers (e.g. condoms or dental dams) during sex and by limiting sexual partners.
- Condoms and dental dams should be used during oral and anal sex, as well.
- Abstaining from sexual intercourse
- Having a sexually monogamous partner who doesn’t have the infection
- To minimize the risk of transmitting gonorrhea to others, avoid having sexual intercourse for at least seven days after completion of treatment.
References
- Murray, P. R., Rosenthal, K. S., & Pfaller, M. A. (2013). Medical microbiology. Philadelphia: Elsevier/Saunders.
- Ng, L. K., & Martin, I. E. (2005). The laboratory diagnosis of Neisseria gonorrhoeae. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale, 16(1), 15–25. doi:10.1155/2005/323082
- https://www.chegg.com/homework-help/laboratory-diagnosis-neisseria-gonorrhoeae-infection-based-m-chapter-3-problem-2ca-solution-9780321802996-exc
- https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-neisseria-gonorrhoeae-infection-in-adults-and-adolescents
- https://apps.who.int/iris/bitstream/handle/10665/267223/PMC2555271.pdf?sequence=1&isAllowed=y
- https://www.healthline.com/health/pregnancy/treatment-chlamydial-infection
- https://microbeonline.com/neisseria-gonorrhoeae-properties-disease-pathogenesis-and-laboratory-diagnosis/
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2015. Atlanta, GA: US Department of Health and Human Services; October 2016.
- Turner CF, Rogers SM, Miller HG, et al. Untreated gonococcal and chlamydial infection in a probability sample of adults. JAMA 2002; 287:726.
- https://www.cdc.gov/std/gonorrhea/

So detailed and well organised
Great piece!