Interesting Science Videos
What is hemicellulose?
Hemicellulose is a group of complex polysaccharides that are found in the fibers of plants along with other polysaccharides like cellulose and pectin.
- Hemicellulose consists of a heterogeneous group of carbohydrates where the structures of these carbohydrates are not clearly understood.
- The structures and physicochemical properties of polysaccharides are very different from each other.
- Hemicelluloses are not structurally related to cellulose, nor do they contain the same building blocks but they are partially soluble in water or alkali.
- Hemicelluloses are mostly mixed polymers, whereas cellulose is a homopolymer of glucose.
- Apart from arabinogalactan, all other hemicelluloses have short side-chains and low molecular weights.
- The hemicelluloses consist of either pentose (xylose, arabinose) or hexoses (glucose, mannose, galactose) as well as uronic acids.
- These polysaccharides adsorb water and function as storage and supporting substances in plants.
- The term ‘hemicelluloses’ is however archaic and various researchers have suggested that it should not be used.
- Alternative terms such as cross-linking glycans have been proposed, but that has other problems since it is not apparent that cross-linking is a major and common feature of the hemicelluloses. This is why the term hemicellulose is still in use.
- Hemicelluloses are grouped into groups that include xyloglucan, xylans, mannans and glucomannans, and β-(1→3, 1→4)-glucans.
- These glycans all have the same equatorial configuration at C1 and C4, and hence the backbones have significant structural similarity.
- Xylan is the representative polysaccharide of this group that is composed of a backbone of β-(1→4)-linked xylose residues. Xylans are also the most abundant carbohydrates after cellulose.
Structure of hemicellulose
Hemicelluloses consist of 50–3000 sugar units as opposed to 7000–15,000 glucose molecules per polymer in cellulose. Hemicellulose is amorphous in structure, not crystalline as cellulose, and therefore more susceptible to hydrothermal extraction and hydrolysis. Hemicelluloses are classified into different groups as xylans, mannans, and glucans on the basis of the primary sugar residue in the backbone.
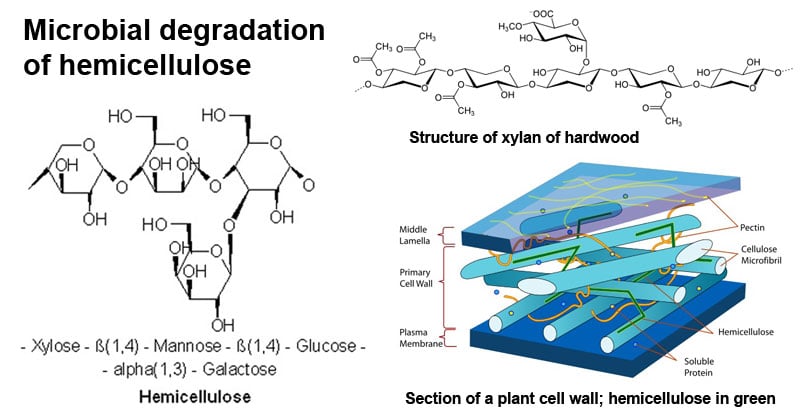
Image Source: Wikipedia.
1. Xylans
- Xylans are a group of polysaccharides consisting of β-(1→4)-linked xylose sugar residues with side branches of α-arabinofructose and α-glucuronic acids that contribute to the cross-linking of cellulose.
- Xylans are categorized into three classes; glucoronoxylan, arabinoxylan, and glucoronoarabinoxylans.
- Xylans does not have a repeated structure, and many variations in the structure are not well known.
- A common feature of xylans is the O-2 linked xylose residues often found as substituents of feruloylarabinofuranosyl side chains.
- Most xylans are acetylated to various degrees, especially in dicot secondary walls. Acetyl groups are attached to O-3 of xylose residues and to a lesser extent to O-2.
2. Mannans
- Mannans are a group of β-(1→4)-linked polysaccharides where the backbones consist entirely of mannose units.
- Mannans have been much studied as a result of their role as seed storage compounds, but they are found in variable amounts in all cell walls.
- Mannnas in yeast consists of α-(1→6)- linked backbone and α-(1→2) and α-(1→3) linked branches.
3. Glucans
- Glucans are polysaccharides composed of glucose units linked by glycosidic bonds. Glycans in hemicellulose are either xyloglucans or β-(1→3,1→4)-glucans.
- Xyloglucans are polysaccharides consisting of β-(1→4)-linked glucan units substituted with xylose molecules.
- β-(1→3,1→4)-glucans are β-(1→4)-linked glucans with interspersed singleβ-(1→3)-linkages.
- These mixed linkage glucans are dominated by cellotriosyl and cellotetrasyl units linked by β-(1→3) linkages, but longer β-(1→4)-linked segments also occur.
What are hemicellulases?
Hemicellulases are a group of enzymes that specifically degrade only hemicelluloses which, in addition to their activity on glycosides, are also frequently capable of hydrolyzing the short-chain or monosaccharide chains from the backbone chain of hemicelluloses.
- The variable structure and organization of hemicellulose require the combined action of many enzymes for its complete degradation.
- The catalytic modules of hemicellulases are either glycoside hydrolases (GHs) that hydrolyze glycosidic bonds, or carbohydrate esterases (CEs), which hydrolyze ester linkages of acetate or ferulic acid side groups.
- Hemicellulases are an important group of enzymes involved in the global carbon cycle where they are a part of the natural waste recycling system.
- Hemicellulases are categorized into four different groups depending on the substrate and linkages they act on; L-arabinanases, D-galactanases, D-mannanases, and D-xylanases.
- Some of these enzymes might even differ in structure and their mode of action.
- Different hemicellulases might even be produced by a different group of organisms like bacteria, fungi, protozoans, algae, and plants.
Microorganisms involved in hemicellulose degradation
1. Hemicellulolytic fungi
- Fungi are among the most active agents of decomposition of organic matter in general, and thus, these microorganisms are the most important group of hemicellulolytic microorganisms.
- Aerobic fungi, such as the fungi Trichoderma and Aspergillus, secrete at high concentrations a large variety of hemicellulases that work synergistically.
- The source of hemicellulases also depends on the type of hemicellulases produced by the microorganisms.
- Besides, other saprophytic fungi like Alternaria solani, Botryosphaeria ribis, Botrytis allii, Corticium centrifugum, Monilia fructigena, Neurospora, Penicillium digitatum, Rhizopus nigricans, Sclerotinia fructigena, etc. are known to produce L-arabinanases and D-mannanases.
- Similarly, fungi like Gibberella saubinetti, Helminthosporium oryzae, Phytophthora infestans, Trametes gibbosa, etc. produce D-galactanases.
- Fungal species found in the marine environments, including Aspergillus sojae, Chaetomium globosum, Agaricus bisporus, Diplodia viticola, Oxiporus, etc. are also considered important sources of hemicellulases.
- Most of the hemicellulases are extracellular with some intracellular hemicellulases.
2. Hemicellulolytic bacteria
- Bacteria also produce different types of hemicellulases either as a singular enzyme or as a multi-enzyme system.
- Most of the bacterial cellulolytic enzymes are reported from Clostridium felsineum, Bacillus subtilis, Acetenobacter mannanolyticus, Bacillus aroideae, Sporocytophaga myxococcoides, etc.
- Besides, rumen bacteria found in various ruminant mammals are also known to produce hemicellulases.
- Some examples include Streptococcus sp., Butyrivibrio fibrisolvens, Ruminococcus albus, Bacteroides ruminicola, etc.
Enzymes involved in the degradation of hemicellulose
Hemicellulases are generally classified into four distinct groups; L-arabinanases, D-galactanases, D-mannanases, and D-xylanases.
1. L-arabinanases
- L-Arabinanases are hydrolytic enzymes capable of degrading L-arabinans, including both of the α-(1→3)-linked L-arabinofuranosyl appendages of the L-arabinan, and the α-(1→5)-linked L-arabinofuranosyl residues of the “linear” chain.
- L-Arabinanases have been reported to be produced by the bacterium Clostridium felsineum var. sikokianum, saprophytic and phytopathogenic fungi, the snail, and plants.
- Rumen bacteria and protozoa and caecal bacteria also produce enzymes capable of liberating L-arabinose from arabinoxylans and are probably a-L-arabinofuranosidases.
- Most L-arabinanases of fungal origin are usually secreted extracellularly into the medium in which the organism is grown, but intracellular L-arabinanases have also been found to exist.
- Several phytopathogenic fungi are known to produce L-arabinanases by induction when grown on media supplemented with L-arabinan, and constitutively when these organisms were grown on D-glucose as the sole carbon source.
2. D-galactanases
- D-galactanases are hydrolytic enzymes capable of degrading D-galactans and L-arabino-D-galactans.
- Two distinct types of D-galactanases are known that are specific for (13) and (14)-β-D-galactopyranosyl linkages.
- Both enzymes are able to degrade D-galactan randomly, to afford D-galactose and D-galactooligosaccharides, and they are therefore endo-D-galactanases.
- D-Galactanases have been reported to be produced by Bacillus subtilis, by a rumen anaerobic bacterium, by fungi, and by plants.
- D-Galactanases are inductive, and those of microbial origin are usually produced extracellularly in response to the carbon source of the culture medium.
3. D-Mannanases
- D-mannanases[( 14)-β-D-mannan mannanohydrolases, endo-D mannanases) are hydrolytic enzymes capable of hydrolyzing the (14)-β-D-mannopyranosyl linkages of D-mannans and D-galacto-D-mannans.
- These enzymes are capable of degrading the D-gluco-D-mannans, D-glucose, D-mannose, and a series of manno- and glucomanno-oligosaccharides.
- D-Mannanases have been reported to be produced by various species of bacteria, including bacteria from human intestines and the rumen.
- Other sources include the rumen protozoa, various fungi (including saprophytic, phytopathogenic, and mycorrhiza fungi), marine algae, germinating terrestrial plant-seeds, and various invertebrates.
4. β-D-xylanases
- β-D-xylanases are hydrolyzing enzyme that degrades β-D-xylans into D-xylose or D-xylose and D-xylo-oligosaccharides.
- Xylanases are of two types that degrade either the (13) linkages and (14) linkages.
- D-Xylanases are known to occur in bacteria from marine and terrestrial environments, fungi (saprophytes, phytopathogens, and mycorrhiza), rumen bacteria and protozoa, ruminant caecal bacteria, insects, snails, crustaceans, marine algae, and germinating seeds of terrestrial plants.
Factors affecting hemicellulose degradation
Degradation of hemicelluloses is affected by a number of factors, some of which are:
1. Temperature and pH
- Temperature and pH both affect the rate of hydrolysis of hemicelluloses. The highest rate of hydrolysis is achieved at pH 6 and 40°C.
- The increase of reaction temperature above 40°C has a negative impact on total xylose production as the temperature probably affects the stability of hemicellulolytic enzymes.
2. Organic matter
- The presence of organic matter also increases the rate of hemicellulose degradation as much of the organic matter act as a substrate.
- However, if hemicellulose is the only component of the matter, the rate of hydrolysis decreases.
- The rate of degradation increases with the addition of a small amount of readily decomposable organic matter as it allows the growth of microorganisms.
3. Dosage of enzymes
- The increase in enzyme concentration also increases the rate of hemicellulose degradation.
- The increase in enzyme concentration increases the number of active sites which subsequently increases the product concentration.
4. Substrate conversion
- The xylose and arabinose production rates decrease rapidly after a few hours of the enzymatic hydrolysis reaction.
- The percentage of arabinose content released during hydrolysis remains significantly lower than the percentage of xylose produced at the same time.
- The sudden fall of the hydrolysis rate is attributed rather to the increasingly limited structural accessibility of the cell wall matrix to enzymes as hydrolysis proceeds than to enzymes’ properties.
Process (Simple Steps) of hemicellulose degradation
The process of hemicellulose degradation is more or less similar to that of homopolymers like cellulose. In the case of hemicelluloses, however, one of the two pathways are followed to obtain the monomeric units.
- The degradation begins with the attack by exoglycocides on the hemicelluloses in order to remove the side-chain substituents, hereby, ‘opening up’ or exposing the backbone glycan chain. This first step allows the glycan chain to be exposed so that it can be easily attacked by the hemicellulases, as a steric hindrance by the side chain residues is reduced.
- Alternatively, the degradation begins with the attack of endohemicellulases on the regions of glycan chain that are unbranched or relatively moderately branched by substituents. It is then followed by the action of endohemicellulases which yields an array of oligosaccharides of a mixed constitution. Both exoglycosidases and endohemicellulases further degrade the resulting fragments.
Read Also: Microbial degradation of cellulose (Enzymes, Steps, Mechanisms)
Mechanisms of microbial degradation of hemicellulose
The mode of action or the mechanism of microbial degradation can only be explained when the enzyme preparations used are homogenous, i.e. a single protein component. The mechanism of microbial degradation is different with different hemicellulases.
1. Xylanases
- D-xylanases of the endo enzyme type is the only xylanases that have been properly characterized.
- These xylanases hydrolyze the 1, 4-β-D-xylopyranosyl linkages of D-glycans such as L-arabino-D-xylans, L-arabino-D-glucoro-D-xylans, and D-glucorono-D-xylans.
- Some of these enzymes might even hydrolyze the (13)-α-L-arabinofuranosyl branch points of arabinoxylan.
- Another group of endo xylanases degrades arabinoxylan and other D-xylans to D-xylose, D-xylooligosaccharides, and in some cases, oligosaccharides containing both L-arabinose and D-xylose.
Example
Bacteria xylanases
- Bacterial xylanases are produced by bacteria like Bacillus and Streptomyces.
- The xylanase preparation from the alkalophile Bacillus degrades arabinoxylan to xylobiose and xylotriose as major end products with smaller amounts of higher xylooligosaccharides.
2. Mannanases
- Mannanases of both the exo and endotypes have been characterized that hydrolyze 1,4-β-D-mannopyranosyl linkages of branched mannans, copolymer mannans, and linear D-mannans.
- The endo-β-mannanases degrade β-D-mannans to D-mannose and a series of mannose oligosaccharides.
- On acid hydrolysis, the enzymic degradation with a β-D-mannosidase yields D-mannose as the only hydrolysis product.
- The preferential attack of endomannanases is on the D-mannose chain at the 3rd and 4th linkages from the nonreducing end of the molecule.
Example
Fungal mannanases
- D-Mannanases of fungal origin have been known to degrade D-mannans in a random manner, and to be of the endotype.
- Mixed oligosaccharides resulting from enzymatic hydrolysis of galactoglucomannans are likely to contain D-galactose in addition to D-glucose and D-mannose.
3. Galactanases
- Galactanases are hydrolytic enzymes that degrade D-galactans and L-arabino-D-galactans.
- Two distinct types of endogalactonases are recognized with a single exo type.
- Endogalactanases degrade the 1,4-β-D-galactosyl linkages of D-galactans randomly to produce D-galactose and galactose oligosaccharides, some of which might contain L-arabinose residues.
Example
Fungal galactonases
- D-galactanases produced by Rhizopus sp. do not act on galactobiose as it is specific only to (13)-β-D-galactopyranosylinkages.
- The enzymes are also capable of removing L-arabinofuranose from arabinogalactosides but do not liberate any L-arabinose from oligosaccharides.
4. Arabinanases
- The action of the L-arabinanae as an endoenzyme yields L-arabinose and an L-arabinose oligosaccharide as major products of hydrolysis.
- Besides, smaller proportions of L-arabinose disaccharides may even be formed.
- L-arabinan-degrading enzymes of the exo-type degrade L-arabinan completely to L-arabinose.
- These enzymes hydrolyze both the (13) and (15)-α-L-arabinofuranosyl residues of L-arabinan.
- Arabinanases hydrolyze both types of linkage of L-arabinan at one active site, and the substrate is attacked from the nonreducing end by a multi-chain mechanism.
- In its attack on L-arabinan, it hydrolyzes the substrate rapidly to the extent of 30%; thereafter, the attack is slow.
- This initial, rapid hydrolysis of L-arabinan corresponds to the favored attack on the (α-L-(13)-linked L-arabinofuranosyl residues, leaving a mainly linear (15)-α-L-arabinan which is slowly, and eventually, completely, hydrolyzed to L-arabinose.
References
- Scheller H. V and Ulvskov P. (2010). Hemicelluloses. Annual Review of Plant Biology, 61(1), 263–289. doi:10.1146/annurev-arplant-042809-112315
- Puls, J. (1997). Chemistry and biochemistry of hemicelluloses: Relationship between hemicellulose structure and enzymes required for hydrolysis. Macromol. Symp., 120: 183-196. https://doi.org/10.1002/masy.19971200119
- Dekker, R. F. H. and Richards G. N. (1976). Hemicellulases: Their Occurrence, Purification, Properties, and Mode of Action. Advances in Carbohydrate Chemistry and Biochemistry. 32. 277–352.doi:10.1016/s0065-2318(08)60339-x
- Dekker, Robert. (, 1985). Biodegradation of the Hemicelluloses. 10.13140/2.1.2266.2081.
- Dehority BA. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968;16(5):781-786.
- Xiros C, Katapodis P, Christakopoulos P. Factors affecting cellulose and hemicellulose hydrolysis of alkali-treated brewers spent grain by Fusarium oxysporum enzyme extract. Bioresour Technol. 2011 Jan;102(2):1688-96. DOI: 10.1016/j.biortech.2010.09.108.
- Shallom D, Shoham Y. Microbial hemicellulases. Curr Opin Microbiol. 2003 Jun;6(3):219-28. DOI: 10.1016/s1369-5274(03)00056-0. PMID: 12831897.
- López-Mondéjar R, Zühlke D, Becher D, Riedel K, Baldrian P. (2016). Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci Rep. Published 2016 Apr 29. doi:10.1038/srep25279
- Brunner, G. (2014). Processing of Biomass with Hydrothermal and Supercritical Water. Supercritical Fluid Science and Technology, 395–509.doi:10.1016/b978-0-444-59413-6.00008-x.
Sources
- 5% – https://www.sciencedirect.com/science/article/pii/S006523180860339X
- 1% – https://www.slideshare.net/VTTFinland/s56
- 1% – https://www.sciencedirect.com/topics/materials-science/heteropolymers
- 1% – https://www.sciencedirect.com/topics/chemistry/hemicellulose
- 1% – https://www.sciencedirect.com/science/article/pii/S0960852410016408
- 1% – https://www.sciencedirect.com/science/article/abs/pii/S006523180860339X
- 1% – https://www.deepdyve.com/lp/annual-reviews/hemicelluloses-AOMyZriu45
- 1% – https://patents.justia.com/patent/20190284075
- <1% – https://www.slideshare.net/shabeelpn/fungi-3614402
- <1% – https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/hemicellulose
- <1% – https://www.sciencedirect.com/science/article/pii/S0956053X20301641
- <1% – https://www.sciencedirect.com/science/article/pii/S0079635206800421
- <1% – https://www.researchgate.net/publication/319133858_Purification_and_characterization_of_mejucin_a_new_bacteriocin_produced_by_Bacillus_subtilis_SN7
- <1% – https://www.researchgate.net/publication/26824062_Rice_family_GH1_glycoside_hydrolases_with_b-D-glucosidase_and_b-D-mannosidase_activities
- <1% – https://www.researchgate.net/publication/267626429_Hemicellulases_Their_Occurrence_Purification_Properties_and_Mode_of_Action
- <1% – https://www.researchgate.net/publication/261033414_Bacillus_pumilus_S124A_carboxymethyl_cellulase_a_thermo_stable_enzyme_with_a_wide_substrate_spectrum_utility
- <1% – https://www.researchgate.net/publication/230184674_Xyloglucan_structure_and_post-germinative_metabolism_in_seeds_of_Copaifera_langsdorfii_from_savanna_and_forest_populations
- <1% – https://www.quora.com/What-is-cellulose-and-hemicellulose-What-are-its-functions
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC516307/
- <1% – https://quizlet.com/241899377/usa-test-prep-questions-flash-cards/
- <1% – https://link.springer.com/article/10.1007/s40974-017-0064-9
- <1% – https://iai.asm.org/content/73/6/3197
- <1% – https://esdac.jrc.ec.europa.eu/projects/SOCO/FactSheets/ENFactSheet-03.pdf
- <1% – https://academic.oup.com/femsre/article/23/4/411/610322
- <1% – http://www.mycosphere.org/pdf/Mycosphere_SI_3b_12.pdf
