Lysine iron agar (LIA) is a differential medium used to test organisms for the ability to deaminate lysine or decarboxylate lysine. Lysine deamination is an aerobic process that occurs on the slant of the media. Lysine decarboxylation is an anaerobic process that occurs in the butt of the media. Edwards and Fife designed LIA in 1961 to presumptively identify Salmonella species, including lactose fermenting Salmonella arizonae, which has been implicated in foodborne outbreaks of gastroenteritis.
Interesting Science Videos
Composition of Lysine Iron Agar (LIA)
| Ingredients | Gms/liter |
| Peptone | 5.000 |
| Yeast extract | 3.000 |
| Dextrose (Glucose) | 1.000 |
| L-Lysine | 10.00 |
| Ferric ammonium citrate | 0.500 |
| Sodium thiosulphate | 0.040 |
| Bromocresol purple | 0.020 |
| Agar | 15.000 |
Final pH (at 25°C): 6.7±0.2
Principle of Lysine Iron Agar (LIA)
- Lysine iron agar contains lysine, peptones, a small amount of glucose, ferric ammonium citrate, and sodium thiosulfate.
- The medium has an aerobic slant and an anaerobic butt. The medium is stabbed to the base of the butt and streaked on slant.
- When glucose is fermented, the butt of the medium becomes acidic (yellow).
- If the organism produces lysine decarboxylase, cadaverine is formed. Cadaverine neutralizes the organic acids formed by glucose fermentation, and the butt of the medium reverts to the alkaline state (purple).
- If the decarboxylase is not produced, the butt remains acidic (yellow).
- If oxidative deamination of lysine occurs, a compound is formed that, in the presence of ferric ammonium citrate and a coenzyme, flavin mononucleotide, forms a burgundy color on the slant.
- If deamination does not occur, the LIA slant remains purple. Bromocresol purple, the pH indicator, is yellow at or below pH 5.2 and purple at or above pH 6.8.
Peptone and yeast extract provide essential nutrients. Dextrose is a source of fermentable carbohydrates. Ferric ammonium citrate and sodium thiosulphate are indicators of H2S formation. Cultures that produce hydrogen sulfide cause blackening of the medium due to ferrous sulfide production.
Preparation and Method of Use of Lysine Iron Agar (LIA)
- Suspend 34.56 grams in 1000 ml distilled water.
- Heat to boiling to dissolve the medium completely.
- Dispense into tubes and sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
- Cool the tubes in slanted position to form slants with deep butts.
- With a straight inoculating needle, inoculate LIA by twice stabbing through the center of the medium to the bottom of the tube and then streaking the slant.
- Cap the tube tightly and incubate at 35°-37°C in ambient air for 18 to 24 hours.
- Examine at 18 – 24 and 40 – 48 hours for growth and color changes in tube butt and slant, and for blackening at the apex of slant.
Result Interpretation on Lysine Iron Agar (LIA)
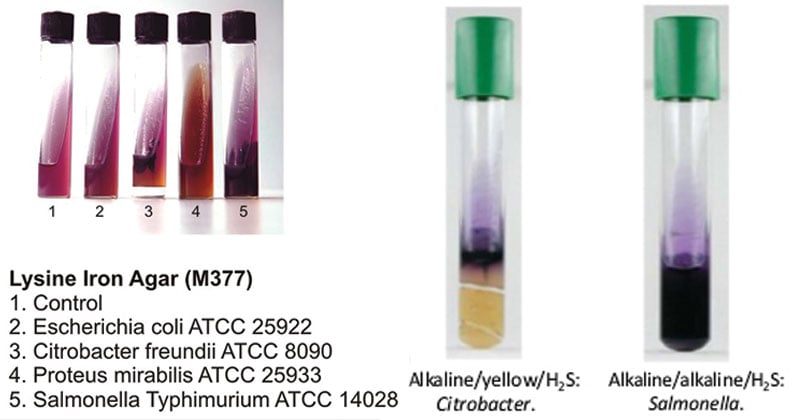
| Organisms | Growth |
| Citrobacter freundii | Luxuriant growth; butt: acidic reaction, yellowing of the medium; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Escherichia coli | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; negative reaction for H2S |
| Proteus mirabilis | Luxuriant growth; butt: acidic reaction, yellowing of the medium; slant: deep red, lysine deamination; positive reaction for H2S, blackening of medium |
| Salmonella Arizonae | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Salmonella Enteritidis | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Salmonella Typhimurium | Luxuriant growth; butt: alkaline reaction, purple or no color change; slant: alkaline reaction, purple or no color change; positive reaction for H2S, blackening of medium |
| Shigella flexneri | Luxuriant growth; butt: acidic reaction, yellowing of the medium; slant: alkaline reaction, purple or no color change; negative reaction for H2S |
Uses of Lysine Iron Agar (LIA)
- It is recommended for the differentiation of enteric organisms based on their ability to decarboxylate or deaminate lysine and to form hydrogen sulfide (H2S).
- This medium is a sensitive medium for the detection of lactose fermenting and lactose non-fermenting Salmonella.
- The agar is particularly useful for distinguishing different Gram-negative bacilli—especially among the Enterobacteriaceae.
- Proteus and Providencia species may be presumptively identified on this medium, as they produce a red slant and an alkaline butt.
Limitations of Lysine Iron Agar (LIA)
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- It is important to stab the butt of the medium. Failure to stab the butt invalidates this test.
- LIA is not as sensitive in detecting hydrogen sulfide in comparison to other iron-containing mediums, such as Sulfide Indole Motility (SIM) Medium and TSIA.
- Proteus sp. that produces hydrogen sulfide will not blacken the medium.
- Certain species or strains may give delayed reactions or completely fail to ferment the carbohydrate in the stated manner.
- Gas production may be irregular or suppressed with organisms other than Citrobacter spp.
References
- http://himedialabs.com/TD/M377.pdf
- http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0381&c=UK&lang=EN
- http://iws2.collin.edu/dcain/CCCCD%20Micro/lysine_iron_agar.htm
- https://microbenotes.com/lysine-iron-agar-test/
- https://assets.thermofisher.com/TFS-Assets/MBD/Instructions/IFU453772.pdf
- http://foodsafety.neogen.com/pdf/acumedia_pi/7211_pi.pdf

Good notes, keep it up