Interesting Science Videos
Structure of Lassa Virus
- The virus is a single-stranded RNA virus belonging to the virus family Arenaviridae.
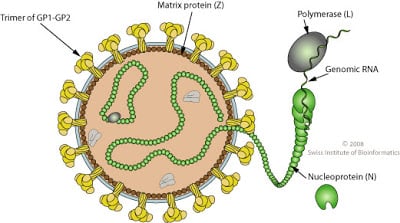
Figure: Structure of Lassa Virus, Source: Swiss Institute of Bioinformatics
- The virion is spherical particles with an average diameter of 90–110 nm.
- Lassa virus is a single-stranded RNA virus that is enveloped in lipid with glycoprotein spikes protruding from the outside surface.
- Glycoproteins on the surface of the virion form T-shaped spikes extending 7–10 nm from the envelope.
Genome of Lassa Virus
- It contains two species of RNA called the small and large units and each unit has two genes at opposite ends that do not overlap.
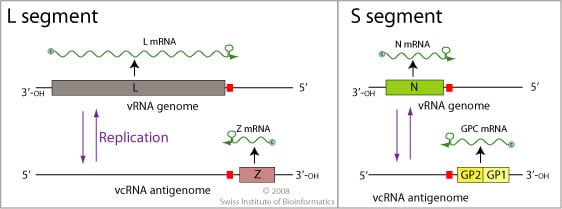
Figure: Genome of Lassa Virus, Source: Swiss Institute of Bioinformatics
- The small unit has some double stranded areas that form stem-loop structures.
- The large species of RNA encodes for the Z and L proteins at the 5’ and 3’ ends respectively and the small species of RNA encodes for glycoprotein and nucleoprotein at the 5’ and 3’ ends respectively.
- Lassa virus consists of four lineages, which have a strain variation of 27% in relation to their nucleotides and 15% in relation to their amino acids.
- The large segment encodes a small zinc-bindingprotein (Z) that regulates transcription and replication and the RNA polymerase (L).
- The small segment encodes the nucleoprotein(NP) and the surface glycoprotein precursor (GP, also known as the viral spike), which is proteolytically cleaved into the envelope glycoproteins GP1 and GP2 that bind to the alpha-dystroglycan receptor and mediate host cell entry.
- The gene that encodes for the nucleoprotein is 1,710 nucleotides long and the protein has 569 amino acids.
- The gene that encodes for the glycoprotein is 1,473 nucleotides long.
Epidemiology of Lassa Virus
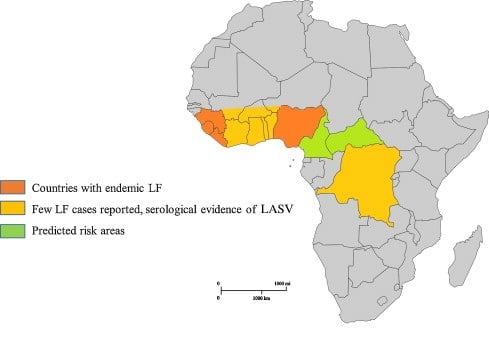
Figure: Epidemiology of Lassa Virus, Source: CDC
- The Lassa virus is so named because, in 1969, it was first isolated and correlated as the causative agent of Lassa fever in a small town called Lassa in North-eastern Nigeria.
- Lassa fever is endemic in parts of west Africa including Sierra Leone, Liberia, Guinea and Nigeria; however, other neighboring countries are also at risk, as the animal vector for Lassa virus, the “multimammate rat” (Mastomys natalensis) is distributed throughout the region.
- Lassa virus consists of four lineages, three of these lineages are located in Nigeria, while the other can be found in Guinea, Liberia, and Sierra Lione.
- The number of Lassa virus infections per year in West Africa is estimated at 100,000 to 300,000 with approximately 5,000 deaths.
- In some areas of Sierra Leone and Liberia, it is known that 10%-16% of people admitted to hospitals every year have Lassa fever, which indicates the serious impact of the disease on the population of this region.
Transmission of Lassa Virus
- Humans contract the virus primarily through contact with the contaminated excreta of Mastomys natalensis rodents (commonly known as the Multimammate rat), which is the natural reservoir for the virus.
- The virus is transmitted to humans through cuts and scratches, or inhaled via dust particles in the air.
- Secondary transmission of the virus between humans occurs through direct contact with infected blood or bodily secretions.
Replication of Lassa Virus
- The Lassa virus gains entry into the host cell by means of the cell-surface receptor the alpha-dystroglycan(alpha-DG), a versatile receptor for proteins of the extracellular matrix.
- After virus enters the cell by alpha-dystroglycan mediated endocytosis, low-pH environment triggers pH-dependent membrane fusion and releases RNP (viral ribonucleoprotein) complex into the cytoplasm.
- Viral RNA is unpacked, and replication and transcription initiate in the cytoplasm.
- As the replication starts, both S and L RNA genomes synthesize the antigenomic S and L RNAs, and from the antigenomic RNAs, genomic S and L RNA are synthesized.
- Both genomic and antigenomic RNAs are needed for transcriptionand translation.
- S RNA encodes GP and NP (viral nucleocapsid protein) proteins, and L RNA encodes Z and L proteins.
- The primary transcription first transcribes mRNAs from the genomic S and L RNAs, which code NP and L proteins, respectively.
- Transcription terminates at the stem-loop (SL) structure within the intergenomic region.
- Arenaviruses use a capsnatching strategy to gain the cap structures from the cellular mRNAs, and it is mediated by the endonuclease activity of the L polymerase and the cap binding activity of NP.
- Antigenomic RNA transcribes viral genes GPC and Z, encoded in genomic orientation, from S and L segments respectively.
- After translation of GPC, it is post translationally modifiedin the endoplasmic reticulum.
- GPC is cleaved into GP1 and GP2 at the later stage of the secretory pathway.
- Cleaved glycoproteinsare incorporated into the virion envelope when the virus buds and release from the cell membrane.
Pathogenesis of Lassa Virus
- When initiating an infection, the Lassa virus attaches to a receptor on the cell surface with the glycoprotein GP-1.
- Its initial sites of replication include dendritic cells and macrophage-monocyte cells and it is then delivered throughout the entire body.
- Infected DC fail to secrete proinflammatory cytokines, do not upregulate costimulatory molecules, such as CD40, CD80, and CD86, and poorly induce proliferation of T cells
- Lassa virus prevents a host’s innate immune systemby NP activity.
- Patients infected with LASV produce IgM and IgG antibody isotypes.
- Neutralizing antibodies appear months after acute infection is resolved, and the titers are often low.
- The neutralizing antibody titers continue to rise even several months after convalescence has been established, which may indicate constant stimulation of B cells due to low levels of virus persistence.
- Antibodies in seroconverted individuals are specific to GPC, NP, and, likely, Z protein.
Clinical manifestations of Lassa Virus
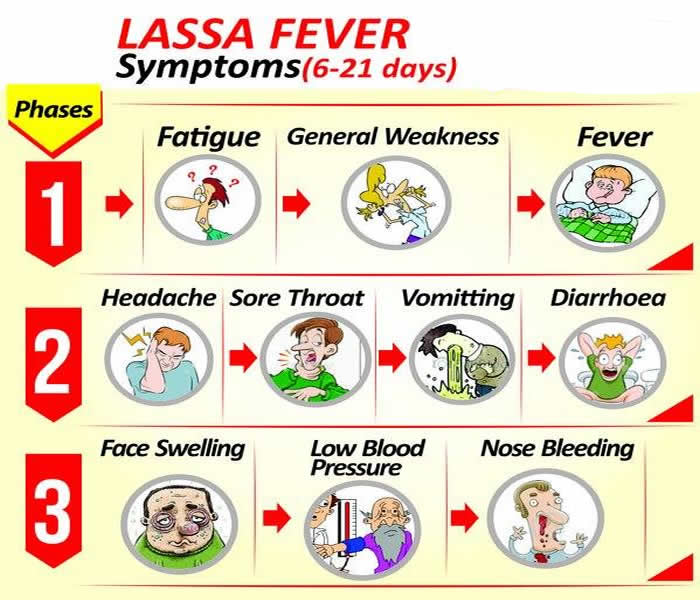
- The incubation period of Lassa fever ranges from 6–21 days.
- Spectrum of clinical effects manifested in Lassa fever range from asymptomatic to multi-organ system failure and death.
- The onset of the disease, when it is symptomatic, is usually gradual, starting with fever, general weakness, and malaise.
- After a few days, headache, sore throat, muscle pain, chest pain, nausea, vomiting, diarrhea, cough, and abdominal pain may follow.
- In severe cases facial swelling, fluid in the lung cavity, bleeding from the mouth, nose, vagina or gastrointestinal tract and low blood pressure may develop.
- Shock, seizures, tremor, disorientation, and coma may be seen in the later stages.
- Death from Lassa fever most commonly occurs 10 to 14 days after symptom onset.
Diagnosis of Lassa Virus
- Detection of IgM and IgG antibodies as well as Lassa antigen by Enzyme-linked immunosorbent serologic assays (ELISA).
- The virus can be uncovered using Reverse transcription PCR (RT-PCR).
- Virus isolation by cell culture, however this procedure should only be done in a high containment laboratory with good laboratory practices. Mice and guinea pigs have been evaluated as models of LASV infection.
- Immunohistochemistry, performed on formalin-fixed tissue specimens, can be used to make a post-mortem diagnosis.
Treatment of Lassa Virus
- Ribavirin, is only effective if administered early in infection (within the first 6 days after disease onset).
Prevention and control of Lassa Virus
- No vaccine for Lassa fever is currently available for use in humans.
- Prevention by promoting good “community hygiene” to discourage rodents from entering homes.
- Effective measures include storing grain and other foodstuffs in rodent-proof containers, disposing of garbage far from the home, maintaining clean households and keeping cats.
- Avoiding contact with blood and body fluids while caring for sick persons.
- In health-care settings, staff should always apply standard infection prevention and control precautions when caring for patients, regardless of their presumed diagnosis. These include basic hand hygiene, respiratory hygiene, use of personal protective equipment (to block splashes or other contact with infected materials), safe injection practices and safe burial practices.
