- Immunoglobulin M (IgM) is an antigen receptor on B cells and the first antibody produced in an immune response.
- It is present both on B cells, and as a soluble molecule in the blood.
- Because of its large size (900 kDa), IgM is found primarily in the intravascular space i.e. in the bloodstream and also lymph fluid.
- Serum IgM exists as a pentamer and comprises approximately 10% of normal human serum Ig content. It is the third most abundant human immunoglobulin.
- It predominates in primary immune responses to most antigens and is the most efficient complement fixing immunoglobulin.
- Plasmablasts that reside in the spleen are believed to be the major site of specific IgM production.
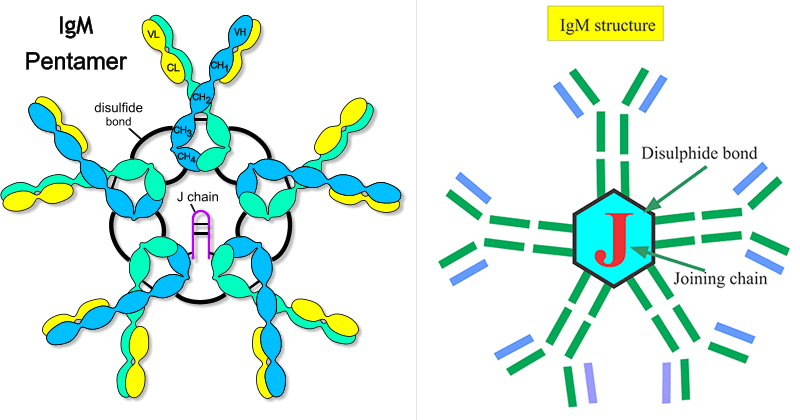
Image Source: Mumtaz khan and Labpedia.
Interesting Science Videos
Structure of IgM
Immunoglobulin M is the third most common serum immunoglobulin and takes one of two forms:
- A pentamer where all heavy chains are identical and all light chains are identical
- A monomer (found on B lymphocytes as B cell receptors)
- On the B cell surface, this molecule is expressed on the plasma membrane as a monomer with a four-chain units – two μ H-chains and two L-chains.
- In this form, it is a B cell antigen receptor, with the H chains each containing an additional hydrophobic domain for anchoring in the membrane.
- In the blood, IgM is composed of five four-chain units (pentamer) held together by disulfide bridges at the carboxy-terminal end of the μ chains.
- Each of the five monomers within the pentamer structure is composed of two light chains (either kappa or lambda) and two heavy chains. However, unlike in IgG, the heavy chain in IgM monomers is composed of one variable and four constant regions, with the additional constant domain replacing the hinge region.
- J-chain is also associated with IgM in the blood and initiates the polymerization of its subunits at the time of its secretion from a plasma cell.
Functions / Significance of IgM
- IgM is the first antibody produced and the prime mediator of the primary immune response.
- Its efficiency in combining with antigen is of particular importance until sufficient quantities of IgG antibody have been synthesized in the body.
- It is responsible for agglutination, neutralizing, and cytolytic reactions and is of vital importance in complement activation and agglutination reactions to combat pathogens.
- IgM interacts with several other physiological molecules such as bind with complement component C1 and activate the classical pathway, binds to the polyimmunoglobulin receptor (pIgR) in a process that brings IgM to mucosal surfaces, such as the gut lumen and into breast milk, etc and have a role in mucosal defense.
- IgM is the first immunoglobulin class to be synthesized by the neonate and plays a role in the pathogenesis of some autoimmune diseases.
- Although IgM antibodies usually have low-affinity binding sites for antigen, they have ten combining sites per molecule that can synergize with each other. It thus has high avidity for antigens and is very efficient per molecule in dealing with pathogens especially early in the immune response.
- Elevated levels of IgM can be a sign of recent infection or exposure to antigen.
References
- https://www.thermofisher.com/np/en/home/lifescience/antibodies/antibodies-learning-center/antibodies-resource-library/antibody-methods/immunoglobulin-igm-class.html
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/immunoglobulin-m
- https://www.bio-rad-antibodies.com/igm-antibodies-immunoglobulin-m.html
- Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers.
- Playfair, J., & Chain, B. (2001). Immunology at a Glance. London: Blackwell Publishing.
- Brooks, G. F., Jawetz, E., Melnick, J. L., & Adelberg, E. A. (2010). Jawetz, Melnick, & Adelberg’s Medical Microbiology. New York: McGraw Hill Medical.
