Interesting Science Videos
Immunoglobulin E (IgE) Definition
Immunoglobulin E (IgE) is one of the 5 classes of immunoglobulins, which is defined by the presence of epsilon (ε) heavy chain. It is present in circulation at very low concentrations of less than 1 µg/mL which is approximately 300-fold lower than that of IgG. Although least abundant, it is in many respects the most potent of the various antibody classes found in mammals. It has a half-life of about 2 days in serum. IgE is produced by IgE plasma cells which are present in mucosal areas, especially in the respiratory tract, where the secreted IgE mediates allergic reactions, and the gastrointestinal tract, where it may mediate expulsion of parasitic worm infestations. Although the physiologic role of IgE is not well characterized it is associated mainly with allergic reactions and thought to be involved in defense against parasites, specifically helminths.
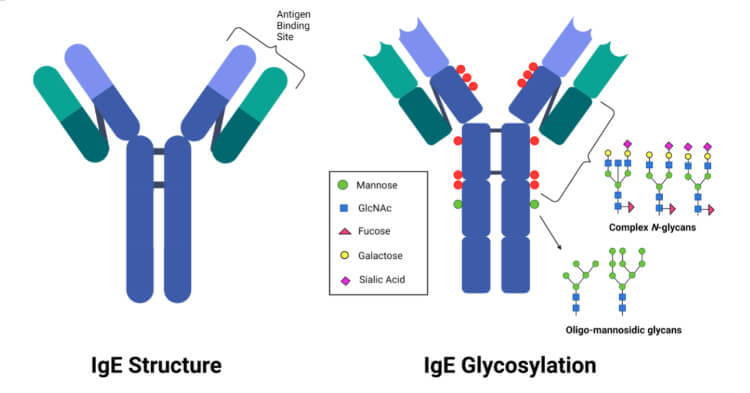
Structure of IgE
- IgE is a glycoprotein produced as a monomeric antibody with two identical heavy chains of the epsilon (ε) class, and two identical Ig light chains. The epsilon (ε) heavy chain has a high (12%) carbohydrate content.
- The structure has two identical antigen-binding areas consisting of both light and heavy chains and valency of 2.
- Heavy and light chains have variable regions on their most N terminal ends.
- Heavy and light chains are subdivided into variable and constant regions. In addition to the disulfide bonds linking the chains together, there are intrachain disulfide links that divide each chain into areas called domains.
- The light chains have two domains, one variable and one constant.
- The heavy chains have five domains, one variable, and four constant-region domains. It is unique in having the additional constant region (CH4).
- CH4 region restricts IgE binding to high-affinity receptors (Fcε-RI) on basophils and mast cells, which contain preformed granules of heparin and histamine.
- A hinge region is absent.
Functions of IgE
The function of IgE is distinct from other immunoglobulins in that it induces activation of mast cells and basophils through the cell-surface receptor Fc epsilon RI which are high-affinity receptors of The Fc region of IgE.
- Allergic reactions are predominantly associated with IgE. Antigen reintroduced into a previously sensitized individual binds to antigen-specific IgE on mast cells and triggers the release of the pharmacologically active agents (e.g., histamine) involved in immediate hypersensitivity syndromes such as hay fever and asthma.
- IgE’s other main functions include providing immunity to parasites such as helminths like Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum.
- IgE also has an essential role in type I hypersensitivity, which manifests in various allergic diseases, such as allergic asthma, most types of sinusitis, allergic rhinitis, food allergies, and specific types of chronic urticaria and atopic dermatitis.
- IgE also plays a pivotal role in responses to allergens, such as anaphylactic drugs, bee stings, and antigen preparations used in desensitization immunotherapy.
- High IgE levels indicate that the body is overreacting to allergens leading to an allergic reaction. It can also be a sign that the body is fighting off an infection from a parasite or with some immune system conditions.
References
- Amarasekera, M. (2011). Immunoglobulin E in health and disease. Asia Pacific Allergy, 1(1), 12–15. http://doi.org/10.5415/apallergy.2011.1.1.12
- https://www.sciencedirect.com/science/article/pii/B9780323069472100094
- https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8159
- Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers.
- Owen, J. A., Punt, J., & Stranford, S. A. (2013). Kuby Immunology (7 ed.). New York: W.H. Freeman and Company.
