Interesting Science Videos
Hopkin’s Cole Test Definition
Hopkin’s Cole test is a specific test used for the detection of indole ring and thus, tryptophan in proteins. The test is also termed as ‘glyoxylic acid test’ as the reagent contains glyoxylic acid. The test was discovered by Frederick Gowland Hopkins and Sydney W. Cole in 1901 as a part of their work on the discovery of tryptophan. Hopkin’s Cole test is extremely similar to the Adamkiewez reaction, which is similar in principle to this test. Hopkin’s Cole test was criticized by Rosenheim, who argued that the glyoxylic acid used in the test could easily decompose into formaldehyde and carbon dioxide at high temperatures. The use of formaldehyde to detect tryptophan was discovered by Rosenheim. Hopkin’s Cole test is one of the color reactions used for the detection of particular amino acids or proteins on the basis of the formation of a specific color. The test detects the indole ring that is found in tryptophan amino acid, which in turn helps in the identification of proteins containing tryptophan.
Objectives of Hopkin’s Cole Test
- To detect the presence of indole ring containing amino acid in proteins.
- To detect the presence of tryptophan-containing proteins.
Principle of Hopkin’s Cole Test
The test is based on the principle that the layering of concentrated sulfuric acid over a mixture of tryptophan-containing proteins with the Hopkin’s Cole reagent results in the formation of a violet ring at the interface. The glyoxylic acid added to the sample combines two tryptophan molecules by acting on the indole ring of the tryptophan molecules. The condensation product thus formed undergoes dehydration to form a violet colored pigment. The color developed in the test is due to the indole group of the tryptophan molecule, which is converted into a colored compound by oxidation brought about by the aldehyde group of glyoxylic acid. The H2SO4 added to the reagent helps to stabilize the glyoxylic acid and prevent its decomposition and the release of carbon dioxide.
Reaction
Tryptophan + Glyoxylic acid → Condensation product + H2O → Violet-coloured compound + H2O
Requirements
Reagent
- Hopkin’s Cole reagent: Glyoxylic acid- It can be prepared by exposing glacial acetic acid to sunlight for a few days.
- Concentrated H2SO4
- Sample: 0.1% amino acid solution
Material Required
- Test tubes
- Test tube stand
- Pipettes
Procedure of Hopkin’s Cole Test
- In a test tube, 2 ml of light-exposed glacial acetic acid and 2 ml of the sample liquid are taken.
- To this, concentrated H2SO4 is added along the sides of the test tube held at a slanting position. Two distinct layers of liquid are to be formed without mixing. (Note: Mouth of the tube must point away from the face, as we should be careful while adding the sulfuric acid.)
- The test tube should be observed for the formation of a colored ring at the interface of two layers.
Result and Interpretation of Hopkin’s Cole Test
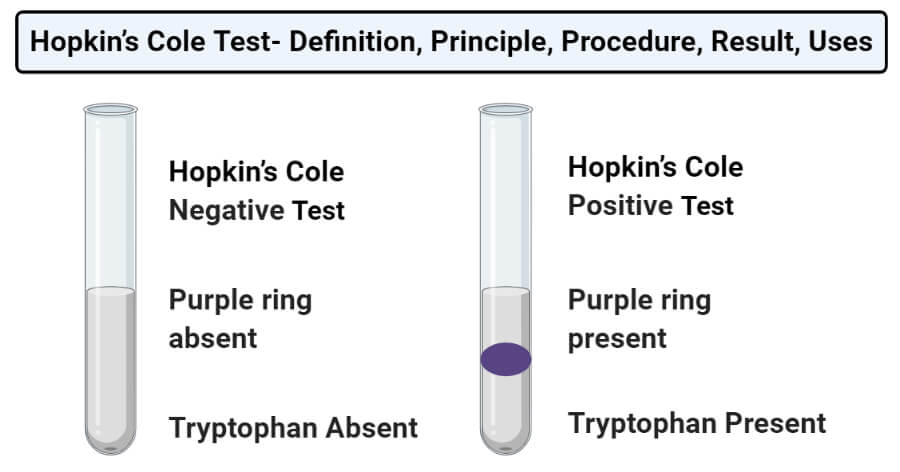
- Positive result: A positive result is represented by the formation of a purple-colored ring at the junction of two layers. This indicates the presence of tryptophan-containing proteins.
- Negative result: A negative result is represented by the absence of a purple-colored ring in the test tube. This indicates the absence of tryptophan-containing proteins.
Uses of Hopkin’s Cole Test
- The test is used for the detection of proteins and amino acids in a sample.
- The test is a simple and easy-to-perform test that helps to identify tryptophan from other amino acids.
Limitations
- Compounds like nitrites, chlorates, nitrates, and excess chlorides prevent the formation of the condensation product.
References
- Nigam S. C. and Omkar (2003). Experimental Animal Physiology and Biochemistry. New Age International Pvt. Limited. New Delhi.
- D (2012). Biochemistry. Fourteenth Edition. Academic Publishers. Kolkata.
- Tiwari, A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- Fearon, W R. “A Study of some Biochemical Tests. No. 2: The Adamkiewicz Protein Reaction. The Mechanism of the Hopkins-Cole Test for Tryptophan. A New Colour Test for Glyoxylic Acid.” The Biochemical journal 14,5 (1920): 548-64. doi:10.1042/bj0140548

In point 2 of Procedure, the mouth of the tube must point away from your face, as you carefully add the sulfuric acid.
Thanks, I have added the precaution as a note in point no. 2 of the procedure. 🙂
Best,
Sagar
Why we add the H2SO4 to the wall of test tube , not to the solution directly??