Interesting Science Videos
Hippurate Hydrolysis Test Definition
Hippurate hydrolysis test is a biochemical test that differentiates microorganisms on the basis of their ability to hydrolyze hippurate into glycine and benzoic acid by the enzymatic action of hippuricase.
- Traditionally, the test was performed using a ferric chloride indicator to detect benzoic acid, which would take a longer period of time.
- Nowadays, however, the test has been modified and is used as a rapid test by the detection of glycine by the addition of ninhydrin as an indicator.
- The test distinguishes Group B streptococci from Groups A, C, F, and G that cannot hydrolyze sodium hippurate. However, some Group D and very few viridans streptococci might also hydrolyze sodium hippurate.
- The test utilizes the ability of an organism to hydrolyze sodium hippurate. It is one of the few tests that aid in the differentiation of bovine β-hemolytic Group B streptococci, from human β-hemolytic Group B Streptococcus species.
Objectives of Hippurate Hydrolysis Test
- To detect the production of the hippuricase enzyme that hydrolyses the substrate hippurate.
- To differentiate bacteria on the basis of their ability to hydrolyze hippurate.
Microorganisms Tested
- Presumptive L. monocytogenes: tiny Gram-positive rods that are catalase-positive, motile at 25°C, and beta-hemolytic.
- Presumptive S. agalactiae: Gram-positive cocci that are catalase-negative, displaying a translucent colony with a characteristic narrow zone of beta-hemolysis.
- Presumptive C. jejuni: curved Gram-negative rods that are oxidase and catalase-positive and do not grow aerobically at 35°C.
- Presumptive G. vaginalis: catalase-negative, tiny Gram-variable rods.
Principle of Hippurate Hydrolysis Test
The hippurate test is based on the ability of the organism to hydrolyze sodium hippurate to benzoic acid and glycine by the action of the enzyme hippuricase. It is primarily used in the identification of Campylobacter jejuni, Listeria monocytogenes, Gardnerella vaginalis, and Streptococcus agalactiae. The ability of bacterial species to hydrolyze hippurate was classically tested using a ferric chloride indicator to detect benzoic acid. However, a 2 hr rapid method, as opposed to the 48 hr traditional method, for the detection of hippurate hydrolysis has since been developed. The rapid test involves ninhydrin as the indicator, which reacts with any protein or amino acid and, in this case, detects glycine. Glycine is deaminated by the oxidizing action of ninhydrin which causes the reduction of ninhydrin, resulting in a purple-colored complex. The test medium used must only have hippurate as a protein source since ninhydrin reacts with any free amino acids present. The rapid hippurate hydrolysis test, which detects the benzoic acid by-product, has been shown to be as sensitive and as specific as the classical method.
Hippurate → Glycine + Benzoic acid
Glycine + Ninhydrin → purple-colored complex
Media, Reagent, and Supplies Used
Reagents Used
a. Hippurate solution
- Hippurate reagent can be found either commercially in the form of dehydrated tubes or as disks or tablets.
- It can be, however, prepared in the laboratory as a 1% hippurate solution.
- For laboratory preparation, 1 g of sodium hippurate is added to 100 ml of distilled water.
b. Ninhydrin
- Ninhydrin reagent can also be found commercially, but it can also be prepared in the laboratory if necessary chemicals are available.
- For laboratory preparation of ninhydrin reagent, 50 ml of acetone and 50 ml of 1-butanol are added to a dark glass bottle. To the bottle, 3.5 grams of ninhydrin is added and mixed well.
Supplies Used
- Sterile wooden sticks or inoculating loops
- Incubator at 35°C
- Test tubes
- Distilled water
Procedure of Hippurate Hydrolysis Test
A. Preparation of hippurate solution
- In the case of commercially bought dehydrated hippurate, 0.2 ml (3 or 4 drops) of distilled water at a pH of 6.8 to 7.2 is added to the test reagent.
- 2 drops of distilled water are added to an empty tube for disk or tablet tests.
- For laboratory prepared hippurate solution, 0.4 ml of the reagent is added to a tube per test.
B. Hydrolysis Test
- In the tube, a heavy suspension (equivalent to no. 3 McFarland standard) is prepared from an 18- to 24-hr culture. The colony should be picked up carefully as not to pick up agar, which contains protein.
- The tube is then incubated for 2 h at 35°C to 37°C.
- After the 2-h incubation period, 2 drops of the ninhydrin solution are added to the hippurate reagent-organism mixture. An additional 2 drops are to be added if the test has 0.4 ml or more of the hippurate.
- The tubes are then reincubated at 35 to 37°C for 30 min.
- The observation of the tubes is made at 10-min intervals for the appearance of deep blue color. The color change will usually appear within 10 to 15 min after the ninhydrin indicator solution has been added.
Result Interpretation of Hippurate Hydrolysis Test
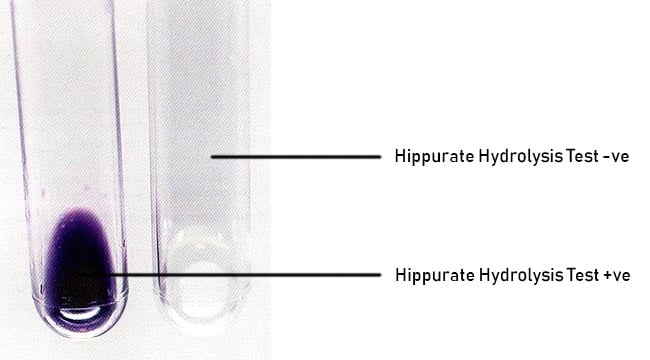
- A positive hippurate hydrolysis reaction is indicated by the appearance of a deep blue color (about the color of crystal violet) within 30 min.
- A negative reaction is indicated by the appearance of a faint purple color or no color change.
Control organisms
- Streptococcus agalactiae: hippurate positive.
- Streptococcus pyogenes: hippurate negative.
Reporting results
- L. monocytogenes organisms are tiny Gram-positive rods that are catalase-positive, motile at 25°C, beta-hemolytic, CAMP positive, and hippurate positive.
- S. agalactiae organisms are catalase-negative, Gram-positive cocci that are identified by having a characteristic narrow zone of beta-hemolysis and are hippurate positive.
- C. jejuni organisms are curved, Gram-negative rods that are identified by having a positive oxidase and catalase reaction, having no growth aerobically at 35°C, and being hippurate positive.
- G. vaginalis organisms are catalase-negative, Gram-variable rods that are hemolytic on human blood agar and are hippurate positive.
Uses of Hippurate Hydrolysis Test
- The hippurate hydrolysis test is used to differentiate between the species of the genus Streptococcus as Group B streptococci can thus be differentiated from Groups A, C, F, and G, members of which cannot hydrolyze sodium hippurate.
- The test is also conducted to detect the production of the hippuricase enzyme that hydrolyzes the substrate hippurate.
- Hippurate hydrolysis by bacteria is also used in the presumptive identification of Gardnerella vaginalis, Campylobacter jejuni, and Listeria monocytogenes.
Limitations of Hippurate Hydrolysis Test
- Not all S. agalactiae organisms are beta-hemolytic, and some viridans group streptococci can be hippurate positive; thus, another test must be done on nonhemolytic colonies to confirm the identification.
- A small number of enterococci are beta-hemolytic and may hydrolyze hippurate, but they are pyrrolidonyl-β-naphthylamide (PYR) positive.
- A small percentage of C. jejuni organisms are hippurate negative and must be identified by other methods.
- A negative test does not rule out the identification of G. vaginalis, since the biotypes that cause bacterial vaginosis can be hippurate negative.
- False-positive results can occur if incubation with ninhydrin exceeds 30 min.
- The hippurate reagent deteriorates in about 7 days when kept at 4°C.
- The inoculation of low inoculum might result in false-negative results.
- The test should not be taken as a confirmatory test, and other immunological, molecular, or mass spectrometry testing must be performed to identify an organism accurately.
References and Sources
- Biochemical Tests for the Identification of Aerobic Bacteria. (2016). Clinical Microbiology Procedures Handbook, 3.17.1.1–3.17.48.3.
- Hippurate Hydrolysis Test. DD035. HiMedia Laboratories.
- Evaluation of the Rapid Hippurate Hydrolysis Test with Enterococcal Group D Streptococci. Journal of Clinical Microbiology Aug 1977, 6 (2) 185.
- 3% – https://www.onlinebiologynotes.com/hippurate-hydrolysis-test-principle-procedure-result-interpretations-and-limitations/
- 2% – https://hypothes.is/users/ddeltoro
- 1% – https://quizlet.com/215783683/microbiology-flash-cards/
- <1% – https://www.slideshare.net/RaviKantAgrawal/biochemical-tests-for-identification-of-bacteria
- <1% – https://www.sciencedirect.com/topics/medicine-and-dentistry/rapid-diagnostic-test
- <1% – https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/rapid-methods
- <1% – https://www.flinnsci.com/api/library/Download/18ce587821c24fb3b0ad7d878bd6a3d9
- <1% – https://www.cdc.gov/streplab/other-strep/general-methods-section1.html
- <1% – https://quizlet.com/57647509/micro-101-wt4-cram-study-flash-cards/
- <1% – https://quizlet.com/39954658/catalase-negative-gram-positive-cocci-flash-cards/
- <1% – https://quizlet.com/367754149/micro-exam-1-combined-set-vc-flash-cards/
- <1% – https://quizlet.com/34476160/gram-positive-organisms-flash-cards/
- <1% – https://quizlet.com/250887902/clin-micro-final-review-flash-cards/
- <1% – https://quizlet.com/222537379/harr-74-microbiology-miscellaneous-and-fastidious-gram-negative-rods-flash-cards/
- <1% – https://microbiologynotes.com/hippurate-hydrolysis-test-principle-uses-procedure-result-interpretation-with-limitations/
- <1% – https://microbeonline.com/hippurate-hydrolysis-test-principle-procedure-uses-results/
- <1% – https://microbenotes.com/bile-solubility-test-principle-procedure-and-result-interpretation/
