Interesting Science Videos
Structure of Herpes simplex virus 1 (HSV-1)
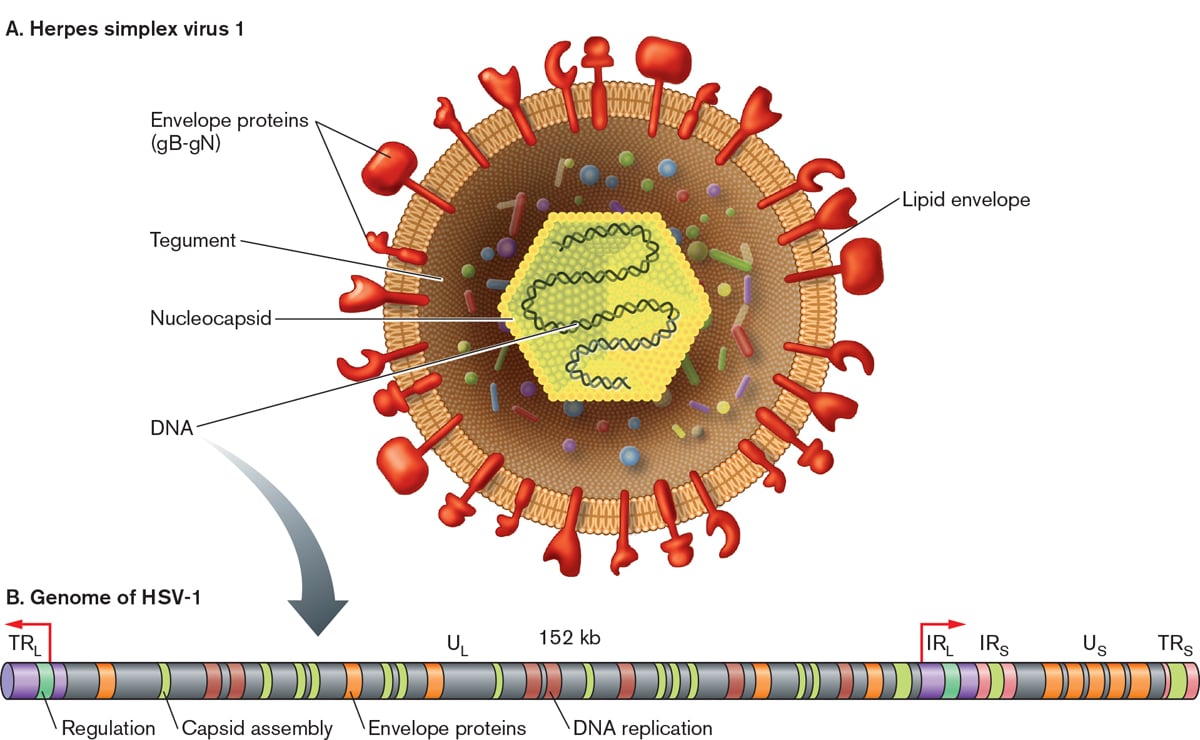
- Herpesviruses are large (150-200 nm size), spherical in shape with icosahedral symmetry.
- The icosahedral protein capsid with average diameter 100 nm consists of 162 hollow hexagonal and pentagonal capsomeres with an electron-dense core containing the double stranded DNA genome with 125-240 kbp nucleotides together forming the nucleocapsid.
- The nucleocapsid is surrounded by an envelope which is lipoprotein in nature.
- Lipid part is derived from the nuclear membrane of the infected host cell.
- Projecting from the trilaminar lipid host-derived envelope are spikes of viral glycoproteins, 8nm long, which bind to specific host receptor and mediate virus entry.
- HSV encodes for at least 11 glycoproteins that serve as (a) viral attachment proteins (gB, gC, gD, gH), (b) fusion proteins (gB), (c) structural proteins, (d) immune escape proteins (gE, and gI), and (e) other fractions.
- In mature virus particles, outside the capsid is an amorphous proteinaceous layer, the tegument, surrounded by a lipid envelope derived from host cell membranes.
- The tegument consist of enzymes such as VP16 which is responsible for subverting cellular proteins and enzymes to involve in viral nucleic acid replication and VHS (Virion Host Shut off ) protein which shut off the host cell protein synthesis in the cytoplasm.
Genome of Herpes simplex virus 1 (HSV-1)
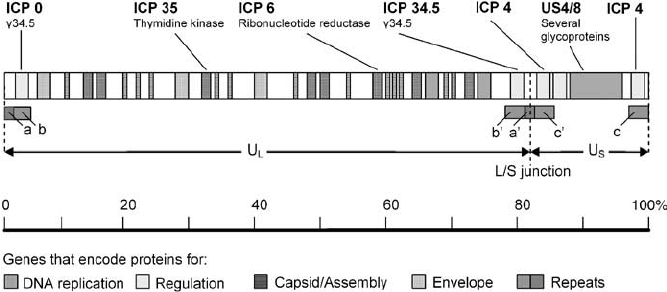
Figure: Map of the genome of herpes simplex virus. The HSV-1 genome is a single, linear molecule of double-stranded DNA approximately 152,000 bp in length. It is divided into two unique segments called long (U L ) and short (U S ). Short regions of repeated sequence (a/b/c and a’/b’/ c’) occur at the genome ends and between the L and S segments. As DNA is replicated, the L and S segments invert at a high rate creating a total of four genome isomers. The four occur at equal frequencies in most wild-type HSV-1 populations. Source: DOI: 10.1038/sj.cgt.7700771
- The virus contains double-stranded DNA genome and is linear with molecular weight of 125–240 kbp.
- The herpesvirus genome is large comprising of 60- 120 genes and encodes at least 100 different proteins.
- Of these, more than 35 polypeptides are involved in the structure of the virus particle and at least 10 are part of the viral envelope.
- Herpesviruses encode an array of virus-specific enzymes involved in nucleic acid metabolism, DNA synthesis, gene expression, and protein regulation (DNA polymerase, helicase-primase, thymidine kinase, transcription factors, protein kinases).
- A striking feature of herpesvirus DNAs is their sequence arrangement possessing terminal and internal repeated sequences.
- On the basis of sequence arrangement it is divided into 6 types – A,B,C,D,E and F.
- Type E genome is found in Herpes Simplex virus.
- The termini of class E consist of two elements.
- The terminal sequences (ab and ca) are inserted in an inverted orientation separating the unique sequences into long (Ul) and short (Us) domains.
Epidemiology of Herpes simplex virus 1 (HSV-1)
- Herpes simplex viruses are worldwide in distribution, equally between the sexes, and without seasonal variation.
- HSV-1 infection is more common than HSV-2 infection with 65% of persons in the United States having antibodies to HSV-1.
- The epidemiology in Europe is similar, with at least half of the population seropositive for HSV-1.
- In the developing world, HSV-1 is almost universal, and usually acquired from intimate contact with family in early childhood.
Transmission of Herpes simplex virus 1 (HSV-1)
- HSV-1 infection is transmitted orally through saliva.
- It is usually transmitted by oral contact, such as by kissing or by sharing of the toothbrushes or other saliva-contaminated items.
- The HSV infection can also occur following mouth-to-skin contact, with the virus entering through minor abrasions in the skin.
- Autoinoculation may also cause infection of the eye.
Replication of Herpes simplex virus 1 (HSV-1)
Source: Wikipedia
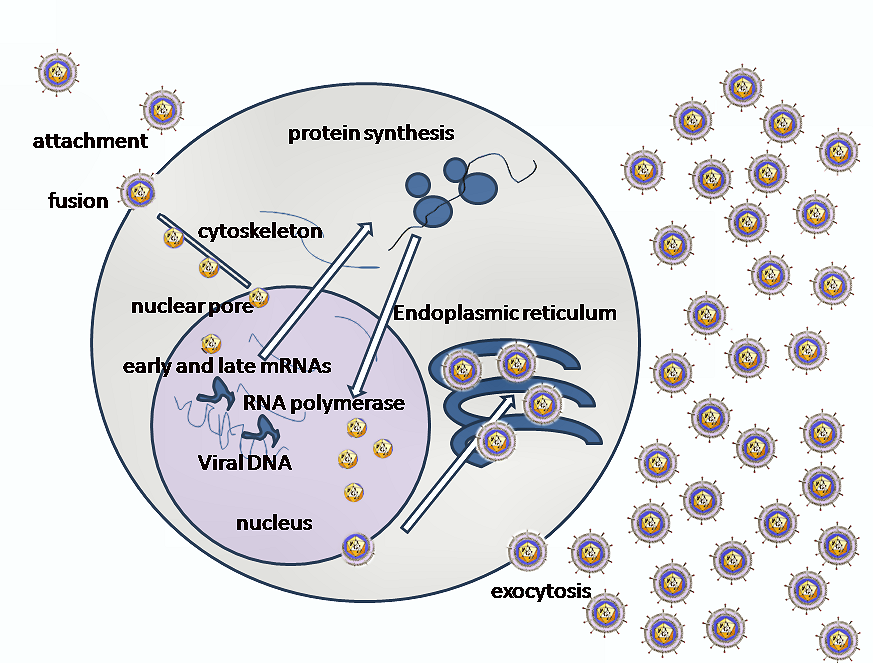
- Invasion of cells by HSV1 requires binding of the envelope gC (glyco-protein-C) and/or gB to Heparan sulfate receptors, engagement by gD of one of several co-receptors including HveA (Herpes virus entry mediator A, also known as HVEM, Herpes Virus Entry Mediators), fusion of the viral envelope with the cell plasma membrane and delivery of the viral capsid into the cell cytoplasm.
- Along with the capsid viral proteins VHS and VP16 are also released in the cytoplasm.
- The incoming viral capsids are subsequently propelled to nucleopore for entry in the nucleus where it gets disintegrated and only DNA is released into the nucleus.
- The viral genome is uncoated for viral transcription and replication in the nucleoplasm.
- There are two main phases of transcription–early, which takes place prior to genome replication, and late, which takes place upon replicated genomes in virus replication compartments formed in the infected cell nucleus.
- Three distinct classes of mRNAs are made: Alpha, Beta, and Gamma which are regulated in a coordinated, cascade fashion.
- The Alpha or IE (Immediate-early) genes contain the major transcriptional regulatory proteins and their production is required for the transcription of the Beta and Gamma gene classes.
- The Beta proteins include the enzymes that are required for replication of the viral genome: a DNA polymerase, a single-strand DNA-binding protein, a primosome or helicase-primase, an origin-binding protein, and a set of enzymes involved in DNA repair and in deoxynucleotide metabolism.
- Viral DNA synthesis begins shortly after the appearance of the Beta proteins and the temporal program of viral gene expression ends with the appearance of the Gamma or late proteins, which constitute the structural proteins of the virus.
- The linear 153Kb pair genome circularizes shortly after infection of susceptible host cells and then enters a rolling circle mode of DNA replication generating branched concatameric DNA, which is then cleaved to release linear ds DNA.
- Viral transcription and DNA replication occurs in the nucleus; the particle assembles and exits from epithelial cells in the skin causing a primary infection.
- The virion acquires its envelope by budding through the nuclear membrane.
Pathogenesis of Herpes simplex virus 1 (HSV-1)
- HSV-1 is spread by kissing or exchanging saliva.
- The virus is usually acquired in childhood or during sexual activity, either through oral–oral or oral–genital contact.
- HSV-1 infects epithelial cells and infection begins with the attachment of virus particles to susceptible cells.
- Virions interact with specific cell-surface receptors through glycoproteins that project from the viral envelope.
- The typical lesion produced by HSV is the vesicle, a ballooning degeneration of intra-epithelial cells, which contains infectious fluid.
- The base of the vesicle contains multinucleate cells (Tzanck cells) and infected nuclei contain eosinophilic inclusion bodies.
- The roof of the vesicle breaks down and an ulcer forms.
- This happens rapidly on mucous membranes and non-keratinizing epithelia; on the skin, the ulcer crusts over, forming a scab, and then heals.
- Natural killer (NK) cells play a significant role in early defenses by recognizing and destroying HSV-infected cells.
- HSV shows three unique biological properties: neurovirulence, latency, and reactivation.
- After the infection at the local site of incoluation virus then invades the local nerve ending; and is transported by retrograde axonal flow to the dorsal root ganglia, where it replicates further, and then undergoes latency.
- Primary HSV infections are usually mild; in fact most are asymptomatic.
- Latency means non replicating state and undergoes in trigeminal ganglia.
- HSV does not replicate in latent stage except for a small RNA, called micro-RNA (encoded by a latency- associated viral gene) which maintains the latent infection and prevents cell death.
- Reactivation processes are still not clearly understood.
- It is suggested that HSV DNA passes along the nerve axon back to the nerve ending where infection of epithelial cells may occur.
- Not all reactivation will result in a visible lesion; there may be asymptomatic shedding of virus only detectable by culture or DNA detection methods.
- The factors influencing the development of recrudescent lesions are not yet clearly identified.
- An increase of CD8+ T suppressor lymphocyte activity is common at the time of recurrences.
- Some mediators (e.g. prostaglandins), and a temporary decrease in immune effector cell function, particularly delayed hypersensitivity, may enhance spread of HSV.
- Certainly, the known triggers for recurrences are accompanied by a local increase in prostaglandin levels, and depression of cell-mediated immunity predisposes to herpes recurrence.
- It occurs naturally, and can be induced by a variety of stimuli such as ultraviolet light (sunlight), fever, trauma and stress.
- The interval between the stimulus and the appearance of a clinically obvious lesion is 2–5 days; this has been demonstrated regularly in patients undergoing neurological interference with their trigeminal ganglion, a common site of herpes latency.
Clinical manifestations of Herpes simplex virus 1 (HSV-1)
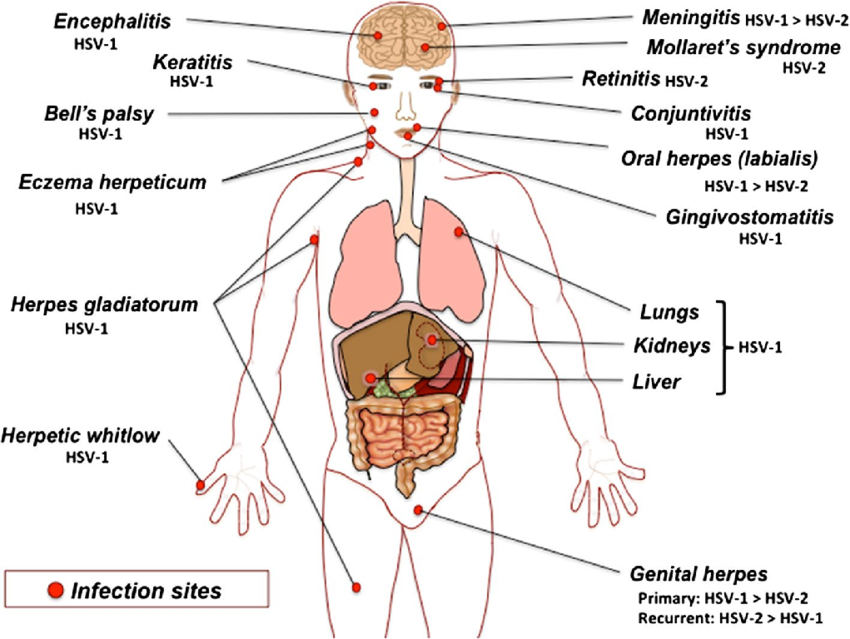
Source: DOI: 10.1007/s00430-014-0358-x
A. Oropharyneal disease
- Primary HSV-1 infections are usually asymptomatic.
- Symptomatic disease occurs most frequently in small children (1–5 years of age) and involves the buccal and gingival mucosa of the mouth.
- Vesicular lesions ulcerate rapidly and are present in the front of the mouth and on the tongue (stomatitis).
- Gingivitis (swollen, tender gums) is the most striking and common lesion.
- Primary infections in adults commonly cause pharyngitis and tonsillitis.
- Vesicles may also develop on the lips and skin around the mouth (herpetic dermatitis), and cervical lymphadenopathy can occur.
- Recurrent disease is characterized by a cluster of vesicles most commonly localized at the border of the lip.
- Lesions progress through the pustular and crusting stages, and healing without scarring usually completes in 8–10 days.
- The lesions may recur, repeatedly and at various intervals, in the same location.
- The frequency of recurrences varies widely among individuals.
B. Keratoconjunctivitis
- HSV infection of the eye may be periorbital together with conjunctivitis, or keratoconjunctivitis associated with corneal ulceration or as vesicles on the eyelids.
- With recurrent keratitis, there may be progressive involvement of the corneal stroma, with permanent opacification and blindness.
C. Skin infections
- Localized lesions caused by HSV-1 may occur in abrasions that become contaminated with the virus (traumatic herpes).
- These lesions are seen on the fingers of dentists and hospital personnel called as herpetic whitlow but other sites may be involved, on the bodies of wrestlers called herpes gladiatorum as a result of direct skin to skin contact.
D. Eczema herpeticum
- Cutaneous infections are often severe and life threatening when they occur in individuals with disorders of the skin, such as eczema or burns, that permit extensive local viral replication and spread called as eczema herpeticum or Kaposi’s varicelliform eruption.
- Extensive ulceration results in protein loss and dehydration, and viraemia can lead to disseminated disease with severe, even fatal consequences.
E. Meningitis/ encephalitis
- HSV-1 infections are considered the most common cause of sporadic, fatal encephalitis in the United States.
- The disease carries a high mortality rate, and those who survive often have residual neurologic defects.
- Direct infection from the nasal mucosa along the olfactory tract is one of the possibility, but the most likely route is central spread from the trigeminal ganglia.
F. Genital herpes
- The prominent cause of genital herpes is HSV-2, however some clinical episodes of genital herpes are caused by HSV-1.
- Primary genital herpes infections can be severe, with illness lasting about 3 weeks.
- Genital herpes is characterized by vesiculo ulcerative lesions of the penis of the male or of the cervix, vulva, vagina, and perineum of the female.
- The sign and symptoms include pain associated with fever, malaise and dysuria.
G. Neonatal herpes
- HSV infection of the newborn may be acquired in utero, during birth, or after birth.
- The mother is the most common source of infection in all cases.
- Neonatal herpes can be acquired postnatally by exposure to either HSV-1 or HSV-2.
- The most common route of infection for HSV to be transmitted to a newborn during birth is by contact with herpetic lesions in the birth canal.
- To avoid infection, delivery by cesarean section has been used in pregnant women with genital herpes lesions.
Laboratory Diagnosis of Herpes simplex virus 1 (HSV-1)
Specimens: vesicle swab, skin swab, vesicle fluid, corneal scrapings, skin scrapings, oral swab, blood, tissue, CSF
Culture
- Inoculation of tissue cultures is used for viral isolation.
- HSV is easy to cultivate, and cytopathic effects usually occur in only 2–3 days.
- Virus can rapidly grow in cell cultures of fibroblasts and epithelial types where the virus produces characteristics grounding and ballooning of cell.
- The agent is then identified by neutralization test or immunofluorescence staining with specific antiserum.
Cytopathology
- Cytopathology involves the detection of multinucleated giant cells in scrapings obtained from the base of vesicle by staining with Giemsa or Wright’s stain, commonly called as Tzanck smear preparation.
- Demonstration of typical giant cells or cow dry type A intranuclear inclusion bodies in the stained smear is diagnostic of HSV infection.
Antigen detection
- The antigen can be detected in vesicle fluid, tissue smear and biopsy by direct fluorescent antigen detection and direct enzyme immunoassay.
Antibody detection
- Antibodies appear in 4-7 days after the infection and peak in 2-4 weeks.
- Primary infection can be detected by determining the presence of IgM or the rising titre of IgG by ELISA, IFT and Complement fixation test.
- Serologic assays based on the type-specific antigens, glycoprotein G, can differentiate between HSV-1 and HSV-2.
Molecular Diagnosis
- Polymerase chain reaction (PCR) is the most sensitive test for detecting HSV DNA and can be used to differentiate between HSV-1 and HSV-2.
Treatment of Herpes simplex virus 1 (HSV-1)
- Acyclovir has a better therapeutic ratio and proven efficacy.
- Acyclovir, a nucleoside analog, is monophosphorylated by the HSV thymidine kinase and is then converted to the triphosphate form by cellular kinases.
- The acyclovir triphosphate is efficiently incorporated into viral DNA by the HSV polymerase, where it then prevents chain elongation.
- Besides acyclovir, valacyclovir, and vidarabine are also used which inhibit DNA synthesis.
Prevention and Control of Herpes simplex virus 1 (HSV-1)
- No vaccination is available for the HSV infection.
- Transmission of herpes simplex can be reduced by:
- alleviating over-crowding
- simple personal hygiene measures
- education regarding the infectious stages
- the use of condoms
